A review of bronchoscopy specimens indicates an unusual number of Mycobacterium fortuitum–positive cultures. Which of the following observations would be the MOST likely cause of this finding?
Bronchoscopes cleaned with sporicidal solution
Inadequate cleaning prior to disinfection
Rinsing with tap water
Drying with air or alcohol
The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies nontuberculous mycobacteria (NTM), including Mycobacterium fortuitum, as organisms commonly associated with water sources, particularly potable water systems. An unusual increase in M. fortuitum–positive bronchoscopy cultures is most often linked to waterborne contamination during endoscope reprocessing, making rinsing with tap water the most likely cause.
Tap water is not sterile and may harbor NTM, which are resistant to standard municipal water treatment and capable of forming biofilms within plumbing systems. If bronchoscopes are rinsed with tap water after high-level disinfection and not followed by appropriate sterile or filtered water rinses and thorough drying, organisms such as M. fortuitum may contaminate internal channels. This can lead to pseudo-outbreaks, where cultures are positive due to contamination rather than true patient infection.
Option B, inadequate cleaning prior to disinfection, can contribute to overall reprocessing failure but is less specifically associated with NTM contamination patterns. Option A is unlikely, as sporicidal solutions are effective disinfectants. Option D, drying with air or alcohol, is a recommended step to reduce microbial growth and would not cause contamination.
For CIC® exam preparation, recognizing that tap water exposure during endoscope reprocessing is a classic source of nontuberculous mycobacteria contamination is a key concept in outbreak investigation and device reprocessing surveillance.
Respiratory tract flora are BEST characterized by which of the following statements?
The airway is sterile below the larynx
Both the upper and lower airways are sterile throughout
Both the upper and lower airways contain small numbers of organisms
The upper airway is heavily colonized while the lower airway is not
The respiratory tract flora refers to the microbial communities inhabiting the respiratory system, and understanding their distribution is essential for infection prevention and diagnosis. The Certification Board of Infection Control and Epidemiology (CBIC) highlights the importance of microbial ecology in the "Identification of Infectious Disease Processes" domain, which aligns with the Centers for Disease Control and Prevention (CDC) and clinical microbiology principles. The question seeks the best characterization of respiratory tract flora, requiring an evaluation of current scientific understanding.
Option C, "Both the upper and lower airways contain small numbers of organisms," is the most accurate statement. The upper respiratory tract (e.g., nasal passages, pharynx) is naturally colonized by a diverse microbial community, including bacteria like Streptococcus, Staphylococcus, and Corynebacterium, as well as some fungi and viruses, acting as a first line of defense. The lower respiratory tract (e.g., trachea, bronchi, alveoli) was traditionally considered sterile due to mucociliary clearance and immune mechanisms. However, recent advances in molecular techniques (e.g., 16S rRNA sequencing) have revealed a low-biomass microbiome in the healthy lower airway, consisting of small numbers of organisms such as Prevotella and Veillonella, likely introduced via microaspiration from the upper tract. The CDC and studies in journals like the American Journal of Respiratory and Critical Care Medicine (e.g., Dickson et al., 2016) support this view, indicating that both regions contain microbial populations, though the lower airway’s flora is less dense and more tightly regulated.
Option A, "The airway is sterile below the larynx," is outdated. While the lower airway was once thought to be sterile, modern research shows a sparse microbial presence, debunking this as a complete characterization. Option B, "Both the upper and lower airways are sterile throughout," is incorrect. The upper airway is clearly colonized, and the lower airway, though low in microbial load, is not entirely sterile. Option D, "The upper airway is heavily colonized while the lower airway is not," overstates the contrast. The upper airway is indeed heavily colonized, but the lower airway is not sterile; it contains small numbers of organisms rather than being completely free of microbes.
The CBIC Practice Analysis (2022) and CDC guidelines on respiratory infections acknowledge the evolving understanding of respiratory flora, emphasizing that both upper and lower airways host small microbial populations in healthy individuals. Option C best reflects this balanced and evidence-based characterization.
What rate is expressed by the number of patients who acquire infections over a specified time period divided by the population at risk of acquiring an infection during that time period?
Incidence rate
Disease specific
Point prevalence
Period prevalence
The incidence rate measures new cases of infection in a population over a defined time period using the formula:
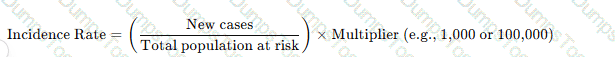
Why the Other Options Are Incorrect?
B. Disease specific – Refers to infections caused by a particular pathogen, not the general rate of new infections.
C. Point prevalence – Measures existing cases at a specific point in time, not new cases.
D. Period prevalence – Includes both old and new cases over a set period, unlike incidence, which only considers new cases.
CBIC Infection Control Reference
APIC defines incidence rate as the number of new infections in a population over a given period.
The degree of infectiousness of a patient with tuberculosis correlates with
the hand-hygiene habits of the patient.
a presence of acid-fast bacilli in the blood.
a tuberculin skin test result that is greater than 20 mm
the number of organisms expelled into the air
The infectiousness of tuberculosis (TB) is directly related to the number of Mycobacterium tuberculosis organisms expelled into the air by an infected patient.
Step-by-Step Justification:
TB Transmission Mechanism:
TB spreads through airborne droplet nuclei, which remain suspended for long periods.
Factors Affecting Infectiousness:
High bacterial load in sputum: Smear-positive patients are much more infectious.
Coughing and sneezing frequency: More expelled droplets increase exposure risk.
Environmental factors: Poor ventilation increases transmission.
Why Other Options Are Incorrect:
A. Hand hygiene habits: TB is airborne, not transmitted via hands.
B. Presence of acid-fast bacilli (AFB) in blood: TB is not typically hematogenous, and blood AFB does not correlate with infectiousness.
C. Tuberculin skin test (TST) >20 mm: TST indicates prior exposure, not infectiousness.
CBIC Infection Control References:
APIC Text, "Tuberculosis Transmission and Control Measures".
Which of the following BEST describes the content of an interpretive surveillance report?
Outlines the organization’s mission for patient quality and safety
Cites the frequency of the evaluation of the monitoring program
Highlights the steps of the facility’s quality improvement activities
Provides findings in a manner designed for the intended audience
The CBIC Certified Infection Control Exam Study Guide (6th edition) explains that an interpretive surveillance report goes beyond simply presenting raw data. Its primary purpose is to translate surveillance findings into meaningful, actionable information that can be understood and used by the intended audience, such as frontline staff, clinical leaders, executive leadership, or quality committees.
Interpretive reports contextualize infection data by explaining trends, comparisons, implications, and recommended actions. This may include highlighting increases or decreases in infection rates, identifying areas of concern, interpreting statistical significance, and linking findings to prevention strategies. The format, level of detail, and language are tailored to the audience’s role and decision-making responsibilities. For example, senior leadership may need high-level summaries and risk implications, while unit-level staff benefit from detailed, practice-focused feedback.
Option A describes a mission statement, not a surveillance report. Option B refers to program evaluation logistics rather than interpretation of findings. Option C outlines quality improvement processes but does not describe how surveillance data are communicated.
For the CIC® exam, it is essential to recognize that interpretive surveillance reporting focuses on meaningful communication, not just data display. Providing findings in a manner designed for the intended audience ensures surveillance data drive prevention actions, accountability, and performance improvement—making option D the best answer.
An infection preventionist has been asked to consult on disinfectant products for use in a long term care home. What should their primary concern be?
Patient care items are cleaned whenever visibly soiled.
An appropriate disinfectant should be available whenever items are used on patients known to be colonized with multi drug resistant organisms.
Disinfectant products should be compatible with the patient care devices used by the facility.
Disinfectant products should have a mild odor to reduce allergy concerns.
The most critical factor in choosing disinfectants in long-term care is compatibility with medical devices to prevent damage and ensure safety. Improper selection can compromise disinfection efficacy and equipment longevity.
The APIC/JCR Workbook highlights:
“Organizations should evaluate compatibility of disinfectant products with the materials used in patient care equipment. Incompatibility can lead to equipment degradation or malfunction”.
This ensures compliance with manufacturer instructions and preserves warranty and functionality.
The infection preventionist recognizes that construction barriers are a key component of the Infection Control Risk Assessment (ICRA). The MOST important factor to consider is that construction barriers should:
Be constructed to withstand normal heating, ventilation, and air conditioning (HVAC) airflow rates.
Provide sealed covers for air intakes and exhausts.
Be able to contain dust or infectious microorganisms generated by the project.
Have walk-off mats that are changed daily.
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that the primary purpose of construction barriers within an Infection Control Risk Assessment (ICRA) is to prevent the dissemination of dust and potentially infectious microorganisms generated during construction, renovation, or maintenance activities. Construction activities can aerosolize fungal spores (such as Aspergillus), bacteria, and other particulate matter that pose a significant risk to immunocompromised patients and other vulnerable populations.
Barriers must therefore be designed and maintained to effectively contain dust and microorganisms at the source, preventing their migration into occupied patient care areas. This containment function is the cornerstone of infection prevention during construction and directly aligns with ICRA goals of risk reduction and patient safety.
While the other options describe supportive or secondary considerations, they are not the most critical factor. Withstanding HVAC airflow (Option A) is important, but it serves the larger goal of containment. Sealing air intakes and exhausts (Option B) is a specific engineering control that may be used as part of containment strategies but does not define the primary purpose of barriers. Walk-off mats (Option D) are useful adjunctive controls but are insufficient alone to prevent airborne transmission of contaminants.
For CIC® exam preparation, it is essential to recognize that containment of dust and infectious agents is the defining function of construction barriers within an ICRA, and all other measures support this central objective.
==========
In a retrospective case-control study, the initial case group is composed of persons
with the disease
without the disease.
with the risk factor under investigation
without the risk factor under investigation
In a retrospective case-control study, cases and controls are selected based on disease status. The case group is composed of individuals who have the disease (cases), while the control group consists of individuals without the disease. This design allows researchers to look back in time to assess exposure to potential risk factors.
Step-by-Step Justification:
Selection of Cases and Controls:
Cases: Individuals who already have the disease.
Controls: Individuals without the disease but similar in other aspects.
Direction of Study:
A retrospective study moves backward from the disease outcome to investigate potential causes or risk factors.
Data Collection:
Uses past medical records, interviews, and laboratory results to determine past exposures.
Common Use:
Useful for studying rare diseases since cases have already occurred, making it cost-effective compared to cohort studies.
Why Other Options Are Incorrect:
B. without the disease: (Incorrect) This describes the control group, not the case group.
C. with the risk factor under investigation: (Incorrect) Risk factors are identified after selecting cases and controls.
D. without the risk factor under investigation: (Incorrect) The study investigates whether cases had prior exposure, not whether they lacked a risk factor.
CBIC Infection Control References:
APIC Text, Chapter on Epidemiologic Study Design.
A facility's goal is to increase hand-hygiene compliance from the current 52% to 75% within 12 months. A gap analysis identifies several different issues. Which of the following is BEST suited for summarizing these issues?
Gantt chart
Flow chart
Ishikawa diagram
Affinity diagram
An Ishikawa diagram (fishbone diagram) is used to visually represent cause-and-effect relationships in problem analysis. It is best for summarizing and categorizing issues found in a gap analysis related to infection prevention.
The APIC Text confirms:
“A fishbone diagram (also called a tree diagram or Ishikawa) allows a team to identify, explore, and graphically display all of the possible causes related to a problem to discover the root cause”.
It’s particularly useful in quality improvement and infection prevention project analysis.
An infection preventionist has decided to perform surveillance for central line–associated bloodstream infections (CLABSIs) in the facility’s ICU. Which of the following is the MOST appropriate denominator to calculate risk-adjusted rates?
Total number of ICU patients
Total number of patients with central lines
Number of patients with infections who have central lines
Number of days patients have central lines in place
The Certification Study Guide (6th edition) emphasizes that device-associated infection rates must be calculated using time-at-risk denominators to accurately reflect patient exposure. For CLABSI surveillance, the most appropriate denominator is central line days, defined as the total number of days patients have one or more central lines in place during the surveillance period.
Using central line days accounts for both the presence and duration of exposure, which is critical for risk adjustment. The longer a central line remains in place, the greater the opportunity for microbial entry and bloodstream infection. This denominator allows for valid trend analysis over time and meaningful benchmarking with national surveillance systems that use standardized definitions and denominators.
The other options are incorrect because they fail to measure exposure accurately. Total ICU patients and total patients with central lines do not account for how long the device was present. Counting only patients who developed infections incorrectly places outcomes in the denominator, which invalidates rate calculations.
The study guide reinforces that numerators represent infection events, while denominators represent populations or time at risk. For CLABSI, the standard rate is expressed as infections per 1,000 central line days, a core concept frequently tested on the CIC exam.
Accurate denominator selection ensures valid surveillance, supports quality improvement efforts, and enables comparison with national benchmarks—making central line days the correct and most appropriate choice.
An outbreak of Candida auris is suspected in the infection preventionist's (IP) facility. The IP's investigation must be conducted in a standard method and communication is critical. Which first step is MOST important?
Conduct environmental cultures
Plan to prevent future outbreaks
Notify facility administration
Perform analytical studies
In an outbreak investigation, the first critical step is to notify facility administration and other key stakeholders. This ensures the rapid mobilization of resources, coordination with infection control teams, and compliance with regulatory reporting requirements.
Why the Other Options Are Incorrect?
A. Conduct environmental cultures – While environmental sampling may be necessary, it is not the first step. The outbreak must first be confirmed and administration alerted.
B. Plan to prevent future outbreaks – Prevention planning happens later after the outbreak has been investigated and controlled.
D. Perform analytical studies – Data analysis occurs after case definition and initial response measures are in place.
CBIC Infection Control Reference
APIC guidelines state that the first step in an outbreak investigation is confirming the outbreak and notifying key stakeholders.
Steam sterilization should be validated with which of the following organisms?
Geobacillus stearothermophilus
Staphylococcus aureus
Bacillus anthracis
Bacillus atrophaeus
The CBIC Certified Infection Control Exam Study Guide (6th edition) clearly states that steam sterilization (moist heat sterilization) must be validated using biological indicators containing Geobacillus stearothermophilus spores. This organism is selected because its spores are highly resistant to moist heat, making them an ideal challenge organism for assessing the effectiveness of steam sterilization processes.
Biological indicators are used to confirm that sterilization conditions—such as temperature, pressure, and exposure time—are sufficient to achieve microbial inactivation. Geobacillus stearothermophilus thrives at high temperatures and demonstrates strong resistance to steam, so if these spores are destroyed, it provides high confidence that other less-resistant microorganisms, including bacteria, viruses, and fungi, have also been eliminated.
The other options are incorrect for steam sterilization validation. Staphylococcus aureus is a vegetative bacterium and is far less resistant than bacterial spores. Bacillus anthracis is not used as a biological indicator due to safety concerns and lack of standardization. Bacillus atrophaeus is used as the biological indicator for dry heat and ethylene oxide sterilization, not steam.
Understanding which biological indicators correspond to specific sterilization modalities is a high-yield topic on the CIC® exam and is essential for ensuring compliance with evidence-based sterilization and disinfection standards.
=======
In order to ensure accurate calculation of central line days, which of the following is TRUE?
Tunneled catheters and ports should be excluded.
A catheter should be in place for longer than 24 hours to be counted.
A patient with more than one device is counted as 1 device day.
Peripheral lines should be included in ICU data.
The CBIC Certified Infection Control Exam Study Guide (6th edition) follows the standardized surveillance methodology used for calculating central line days, which is essential for accurate reporting of central line–associated bloodstream infection (CLABSI) rates. A central line day is counted for each patient who has one or more central lines in place at the time of the daily count, regardless of the number of central lines present.
Therefore, if a patient has more than one central line, the patient is still counted as one central line day, making option C the correct statement. This approach ensures consistency and comparability of CLABSI rates across units and facilities.
Option A is incorrect because tunneled central venous catheters and implanted ports are included in central line counts if they meet the definition of a central line. Option B is incorrect because a central line is counted on any day it is present, even if it has been in place for less than 24 hours. Option D is incorrect because peripheral intravenous lines are not central lines and must never be included in central line day counts.
Accurate calculation of device days is a foundational surveillance competency for infection preventionists. Understanding these definitions is critical for valid CLABSI rate calculation, benchmarking, and performance improvement and is a frequently tested concept on the CIC® exam.
What question would be appropriate for an infection preventionist to ask when reviewing the discussion section of an original article?
Was the correct sample size and analysis method chosen?
Could alternative explanations account for the observed results?
Is the study question important, appropriate, and stated clearly?
Are criteria used to measure the exposure and the outcome explicit?
When reviewing the discussion section of an original article, an infection preventionist must focus on critically evaluating the interpretation of the study findings, their relevance to infection control, and their implications for practice. The discussion section typically addresses the meaning of the results, compares them to existing literature, and considers limitations or alternative interpretations. The appropriate question should align with the purpose of this section and reflect the infection preventionist's need to assess the validity and applicability of the research. Let’s analyze each option:
A. Was the correct sample size and analysis method chosen?: This question pertains to the methodology section of a research article, where the study design, sample size, and statistical methods are detailed. While these elements are critical for assessing the study's rigor, they are not the primary focus of the discussion section, which interprets results rather than re-evaluating the study design. An infection preventionist might ask this during a review of the methods section, but it is less relevant here.
B. Could alternative explanations account for the observed results?: The discussion section often explores whether the findings can be explained by factors other than the hypothesized cause, such as confounding variables, bias, or chance. This question is highly appropriate for an infection preventionist, as it encourages a critical assessment of whether the results truly support infection control interventions or if other factors (e.g., environmental conditions, patient factors) might be responsible. This aligns with CBIC's emphasis on evidence-based practice, where understanding the robustness of conclusions is key to applying research to infection prevention strategies.
C. Is the study question important, appropriate, and stated clearly?: This question relates to the introduction or background section of an article, where the research question and its significance are established. While important for overall study evaluation, it is not specific to the discussion section, which focuses on interpreting results rather than revisiting the initial question. An infection preventionist might consider this earlier in the review process, but it does not fit the context of the discussion section.
D. Are criteria used to measure the exposure and the outcome explicit?: This question is relevant to the methods section, where the definitions and measurement tools for exposures (e.g., a specific intervention) and outcomes (e.g., infection rates) are described. The discussion section may reference these criteria but focuses more on their implications rather than their clarity. This makes it less appropriate for the discussion section specifically.
The discussion section is where authors synthesize their findings, address limitations, and consider alternative explanations, making option B the most fitting. For an infection preventionist, evaluating alternative explanations is crucial to ensure that recommended practices (e.g., hand hygiene protocols or sterilization techniques) are based on solid evidence and not confounded by unaddressed variables. This critical thinking is consistent with CBIC's focus on applying research to improve infection control outcomes.
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain I: Identification of Infectious Disease Processes, which emphasizes critical evaluation of research evidence.
CBIC Examination Content Outline, Domain V: Management and Communication, which includes assessing the validity of research findings for infection control decision-making.
The infection preventionist (IP) is invited to a planning meeting for a new oncology unit. The team is excited about the new design and wants lots of natural plants to be incorporated. What action should the IP take?
Allow the process to continue.
Ask about the air handling unit.
Ask whether artificial plants could be used instead.
Prohibit any discussion on the inclusion of natural plants.
The CBIC Certified Infection Control Exam Study Guide (6th edition) clearly emphasizes that oncology units house highly immunocompromised patients, making environmental sources of infection a critical concern during design and planning phases. Natural plants, soil, and standing water are well-recognized reservoirs for environmental fungi and gram-negative bacteria, including Aspergillus, Fusarium, and Pseudomonas species, all of which pose a serious infection risk to oncology patients.
Rather than allowing the process to continue unchecked (Option A) or completely shutting down discussion (Option D), the infection preventionist’s role is to guide the team toward safer alternatives while supporting collaborative planning. Asking whether artificial plants can be used instead (Option C) is the most appropriate action because it maintains the aesthetic goals of the design team while eliminating the infection risks associated with live plants.
Option B, asking about the air handling unit, is important in oncology design but does not directly address the specific and preventable risk posed by natural plants. The Study Guide notes that potted plants, dried flower arrangements, and soil-containing décor should be avoided in areas caring for severely immunocompromised patients.
For the CIC® exam, this question highlights the IP’s responsibility to anticipate environmental infection risks early in facility planning and recommend practical, evidence-based alternatives that protect patient safety without unnecessarily impeding design goals.
A nutrition support team wants to determine whether patients who receive total parenteral nutrition (TPN) at home are at increased risk of central line–associated bloodstream infection (CLABSI) compared with patients who receive TPN in the hospital. The BEST way to compare these two groups is to calculate the:
Percentage of patients in each group who became infected.
Infections per 1,000 central line days in each group.
Number of infections in each group this year compared to last year.
Ratio of infected to noninfected central lines in each group.
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that accurate comparison of healthcare-associated infection risk between groups requires use of standardized, exposure-based rates. For central line–associated bloodstream infections (CLABSIs), the recommended metric is infections per 1,000 central line days, which accounts for the amount of time patients are actually exposed to the risk factor—in this case, the presence of a central venous catheter.
Patients receiving TPN at home and those receiving TPN in the hospital may differ substantially in duration of catheter use, care practices, and patient acuity. Simply comparing percentages or raw numbers of infections fails to adjust for differences in central line utilization and can result in misleading conclusions. By using central line days as the denominator, infection rates are normalized and allow for valid comparisons between populations and settings.
Option A does not account for differences in exposure time. Option C compares different time periods rather than comparing risk between groups. Option D provides a ratio but lacks standardization and is not consistent with accepted surveillance methodology.
The Study Guide reinforces that device-associated infection surveillance—such as CLABSI monitoring—must use device days to assess true risk and guide prevention strategies. Understanding and applying correct epidemiologic measures is a core competency for infection preventionists and a frequently tested concept on the CIC® exam.
==========
In which of the following ways is human immunodeficiency virus similar to the Hepatitis B virus?
The primary mechanism of transmission for both is maternal-fetal
Needlestick exposure leads to a high frequency of healthcare worker infection
Transmission may occur from asymptomatic carriers
The risk of infection from mucous membrane exposure is the same
The human immunodeficiency virus (HIV) and Hepatitis B virus (HBV) are both bloodborne pathogens that pose significant risks in healthcare settings, and understanding their similarities is crucial for infection prevention and control. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the importance of recognizing transmission modes and implementing appropriate precautions in the "Prevention and Control of Infectious Diseases" domain, aligning with guidelines from the Centers for Disease Control and Prevention (CDC). Comparing these viruses involves evaluating their epidemiology, transmission routes, and occupational risks.
Option C, "Transmission may occur from asymptomatic carriers," is the correct answer. Both HIV and HBV can be transmitted by individuals who are infected but show no symptoms, making asymptomatic carriage a significant similarity. For HBV, chronic carriers (estimated at 257 million globally per WHO, 2019) can transmit the virus through blood, semen, or other bodily fluids without overt signs of disease. Similarly, HIV-infected individuals can remain asymptomatic for years during the latent phase, yet still transmit the virus through sexual contact, blood exposure, or perinatal transmission. The CDC’s "Guidelines for Prevention of Transmission of HIV and HBV to Healthcare Workers" (1987, updated 2011) and "Epidemiology and Prevention of Viral Hepatitis" (2018) highlight this shared characteristic, underscoring the need for universal precautions regardless of symptom status.
Option A, "The primary mechanism of transmission for both is maternal-fetal," is incorrect. While maternal-fetal transmission (perinatal transmission) is a significant route for both HIV and HBV—occurring in 5-10% of cases without intervention for HBV and 15-45% for HIV without antiretroviral therapy—it is not the primary mechanism. For HBV, the primary mode is horizontal transmission through unprotected sexual contact or percutaneous exposure (e.g., needlesticks), accounting for the majority of cases. For HIV, sexual transmission and intravenous drug use are the leading modes globally, with maternal-fetal transmission being a smaller proportion despite its importance. Option B, "Needlestick exposure leads to a high frequency of healthcare worker infection," is partially true but not a precise similarity. Needlestick exposures carry a high risk for HBV (transmission risk ~30% if the source is HBeAg-positive) and a lower risk for HIV (~0.3%), but the frequency of infection among healthcare workers is significantly higher for HBV due to its greater infectivity and stability outside the host. This makes the statement more characteristic of HBV than a shared trait. Option D, "The risk of infection from mucous membrane exposure is the same," is false. The risk of HIV transmission via mucous membrane exposure (e.g., splash to eyes or mouth) is approximately 0.09%, while for HBV it is higher (up to 1-2% depending on viral load and exposure type), reflecting HBV’s greater infectivity.
The CBIC Practice Analysis (2022) and CDC guidelines emphasize the role of asymptomatic transmission in shaping infection control strategies, such as routine testing and post-exposure prophylaxis. This shared feature of HIV and HBV justifies Option C as the most accurate similarity.
Microfiber cloths and mops are preferred over cotton because microfiber:
Is more cost effective.
Is positively charged to better attract dirt.
Can be laundered and dried with other textiles.
Is versatile for both smooth and rough surfaces.
The CBIC Certified Infection Control Exam Study Guide (6th edition) explains that microfiber cleaning materials are preferred over traditional cotton cloths and mops because of their electrostatic properties, which enhance cleaning effectiveness. Microfiber is composed of very fine synthetic fibers that become positively charged, allowing them to attract and trap negatively charged dirt, dust, and microorganisms rather than simply pushing them across surfaces.
This electrostatic attraction enables microfiber to remove a significantly higher percentage of bacteria and organic material from surfaces compared to cotton, even when used with less cleaning solution or disinfectant. The split fiber structure also increases surface area, allowing microorganisms and debris to be captured within the fibers rather than redistributed. These properties make microfiber particularly effective for environmental cleaning in healthcare settings, where surface contamination contributes to transmission of healthcare-associated infections.
Option A is incorrect because microfiber products are often more expensive initially, though they may be cost-effective over time. Option C is incorrect because microfiber must be laundered separately under specific conditions to maintain effectiveness. Option D may be true but is not the primary reason for preference.
For the CIC® exam, it is important to recognize that microfiber’s positive charge and superior ability to attract and retain microorganisms are the key reasons it is favored over cotton for environmental cleaning and infection prevention.
An infection preventionist (IP) observes an increase in primary bloodstream infections in patients admitted through the Emergency Department. Poor technique is suspected when peripheral intravenous (IV) catheters are inserted. The IP should FIRST stratify infections by:
Location of IV insertion: pre-hospital, Emergency Department, or in-patient unit.
Type of dressing used: gauze, CHG impregnated sponge, or transparent.
Site of insertion: hand, forearm, or antecubital fossa.
Type of skin preparation used for the IV site: alcohol, CHG/alcohol, or iodophor.
When an infection preventionist (IP) identifies an increase in primary bloodstream infections (BSIs) associated with peripheral intravenous (IV) catheter insertion, the initial step in outbreak investigation and process improvement is to stratify the data to identify potential sources or patterns of infection. According to the Certification Board of Infection Control and Epidemiology (CBIC), the "Surveillance and Epidemiologic Investigation" domain emphasizes the importance of systematically analyzing data to pinpoint contributing factors, such as location, technique, or equipment use, in healthcare-associated infections (HAIs). The question specifies poor technique as a suspected cause, and the first step should focus on contextual factors that could influence technique variability.
Option A, stratifying infections by the location of IV insertion (pre-hospital, Emergency Department, or in-patient unit), is the most logical first step. Different settings may involve varying levels of training, staffing, time pressure, or adherence to aseptic technique, all of which can impact infection rates. For example, pre-hospital settings (e.g., ambulance services) may have less controlled environments or less experienced personnel compared to in-patient units, potentially leading to technique inconsistencies. The CDC’s Guidelines for the Prevention of Intravascular Catheter-Related Infections (2017) recommend evaluating the context of catheter insertion as a critical initial step in investigating BSIs, making this a priority for the IP to identify where the issue is most prevalent.
Option B, stratifying by the type of dressing used (gauze, CHG impregnated sponge, or transparent), is important but should follow initial location-based analysis. Dressings play a role in maintaining catheter site integrity and preventing infection, but their impact is secondary to the insertion technique itself. Option C, stratifying by the site of insertion (hand, forearm, or antecubital fossa), is also relevant, as anatomical sites differ in infection risk (e.g., the hand may be more prone to contamination), but this is a more specific factor to explore after broader contextual data is assessed. Option D, stratifying by the type of skin preparation used (alcohol, CHG/alcohol, or iodophor), addresses antiseptic efficacy, which is a key component of technique. However, without first understanding where the insertions occur, it’s premature to focus on skin preparation alone, as technique issues may stem from systemic factors across locations.
The CBIC Practice Analysis (2022) supports a stepwise approach to HAI investigation, starting with broad stratification (e.g., by location) to guide subsequent detailed analysis (e.g., technique-specific factors). This aligns with the CDC’s hierarchical approach to infection prevention, where contextual data collection precedes granular process evaluation. Therefore, the IP should first stratify by location to establish a baseline for further investigation.
Which of the following correctly characterizes endovaginal ultrasound probes?
They must be sterilized with a liquid chemical sterilant after use.
They are protected from viral contamination if covered by a sheath.
They are critical items based on the Spaulding Classification System.
They may be contaminated with human papillomavirus pre-examination.
The Certification Study Guide (6th edition) classifies endovaginal ultrasound probes as semi-critical devices because they come into contact with mucous membranes. As such, they require high-level disinfection (HLD) between patients, not sterilization, unless the manufacturer specifically requires it. This immediately eliminates option A, which incorrectly states sterilization is required.
Option B is incorrect because probe covers or sheaths do not eliminate the risk of contamination. Numerous studies referenced in infection prevention literature and reflected in the study guide demonstrate that probe covers can fail, tear, or leak, allowing microorganisms—including viruses—to contaminate the probe surface. Therefore, HLD is required regardless of sheath use.
Option C is incorrect because critical items, by definition, enter sterile tissue or the vascular system. Endovaginal probes contact mucous membranes only and are therefore not critical items under the Spaulding Classification System.
Option D is correct because endovaginal probes may be contaminated with human papillomavirus (HPV) prior to examination, even when probe covers are used. HPV is particularly concerning due to its resistance to some low-level disinfectants and its ability to persist on surfaces. The study guide highlights HPV as a key organism driving strict reprocessing requirements for these probes.
This question reflects a high-yield CIC exam concept: probe covers do not replace high-level disinfection, and viral contamination—including HPV—remains a significant risk.
A hospital is experiencing an increase in vancomycin-resistant Enterococcus (VRE) infections in the hematology-oncology unit. Which of the following interventions is MOST effective in halting the spread of VRE in this high-risk setting?
Screening all patients on admission and placing positive patients in isolation.
Restricting the use of vancomycin for all patients in the hospital.
Implementing a hand hygiene compliance audit and feedback system.
Conducting environmental sampling for VRE contamination weekly.
Comprehensive and Detailed In-Depth Explanation:
Hand hygiene remains the single most effective intervention to prevent the spread of vancomycin-resistant Enterococcus (VRE) in healthcare settings. Implementing an audit and feedback system significantly improves compliance and reduces VRE transmission.
Step-by-Step Justification:
Hand Hygiene Compliance Audit and Feedback (Best Strategy)
Studies show that poor hand hygiene is the primary mode of VRE transmission in hospitals.
Implementing real-time auditing with feedback ensures sustained compliance and helps identify weak areas.
Why Other Options Are Incorrect:
A. Screening all patients and isolating VRE-positive patients:
While screening helps identify carriers, contact precautions alone are not sufficient without strong hand hygiene enforcement.
B. Restricting vancomycin use:
While antimicrobial stewardship is crucial, vancomycin use alone does not drive VRE outbreaks—poor infection control practices do.
D. Conducting environmental sampling weekly:
Routine sampling is not necessary; immediate terminal disinfection and improved hand hygiene are more effective.
CBIC Infection Control References:
APIC Text, "VRE Prevention and Hand Hygiene," Chapter 11.
APIC-JCR Workbook, "Antimicrobial Resistance and Infection Control Measures," Chapter 7.
Which of the following pathogens is associated with the highest risk of seroconversion after percutaneous exposure?
Shigella
Syphilis
Hepatitis A
Hepatitis C
Among the listed pathogens, Hepatitis C has the highest risk of seroconversion following a percutaneous exposure, though it's important to note that Hepatitis B actually has the highest overall risk. However, since Hepatitis B is not listed among the options, the correct choice from the available ones is Hepatitis C.
The APIC Text confirms:
“The average risk of seroconversion after a percutaneous injury involving blood infected with hepatitis C virus is approximately 1.8 percent”.
The other options are not bloodborne pathogens typically associated with high seroconversion risks after needlestick or percutaneous exposure:
A. Shigella – transmitted fecal-orally, not percutaneously.
B. Syphilis – transmitted sexually or via mucous membranes.
C. Hepatitis A – primarily fecal-oral transmission, low occupational seroconversion risk.
Which of the following statements describes the MOST important consideration of an infection preventionist when assessing the effectiveness of an infection control action plan?
Re-evaluate the action plan every three years.
Update the plan before the risk assessment is completed.
Develop a timeline and assign responsibilities for the stated action.
Monitor and validate the related outcome and process measures.
Assessing the effectiveness of an infection control action plan is a critical responsibility of an infection preventionist (IP) to ensure that interventions reduce healthcare-associated infections (HAIs) and improve patient safety. The Certification Board of Infection Control and Epidemiology (CBIC) highlights this process within the "Surveillance and Epidemiologic Investigation" and "Performance Improvement" domains, emphasizing the need for ongoing evaluation and data-driven decision-making. The Centers for Disease Control and Prevention (CDC) and other guidelines stress that the ultimate goal of an action plan is to achieve measurable outcomes, such as reduced infection rates, which requires systematic monitoring and validation.
Option D, "Monitor and validate the related outcome and process measures," is the most important consideration. Outcome measures (e.g., infection rates, morbidity, or mortality) indicate whether the action plan has successfully reduced the targeted infection risk, while process measures (e.g., compliance with hand hygiene or proper catheter insertion techniques) assess whether the implemented actions are being performed correctly. Monitoring involves continuous data collection and analysis, while validation ensures the data’s accuracy and relevance to the plan’s objectives. The CBIC Practice Analysis (2022) underscores that effective infection control relies on evaluating both outcomes (e.g., decreased central line-associated bloodstream infections) and processes (e.g., adherence to aseptic protocols), making this a dynamic and essential step. The CDC’s "Compendium of Strategies to Prevent HAIs" (2016) further supports this by recommending regular surveillance and feedback as key to assessing intervention success.
Option A, "Re-evaluate the action plan every three years," suggests a periodic review, which is a good practice for long-term planning but is insufficient as the most important consideration. Infection control requires more frequent assessment (e.g., quarterly or annually) to respond to emerging risks or outbreaks, making this less critical than ongoing monitoring. Option B, "Update the plan before the risk assessment is completed," is illogical and counterproductive. Updating a plan without a completed risk assessment lacks evidence-based grounding, undermining the plan’s effectiveness and contradicting the CBIC’s emphasis on data-driven interventions. Option C, "Develop a timeline and assign responsibilities for the stated action," is an important initial step in implementing an action plan, ensuring structure and accountability. However, it is a preparatory activity rather than the most critical factor in assessing effectiveness, which hinges on post-implementation evaluation.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize outcome and process monitoring as the cornerstone of infection control effectiveness, enabling IPs to adjust strategies based on real-time evidence. Thus, Option D represents the most important consideration for assessing an infection control action plan’s success.
During the past week, three out of four blood cultures from a febrile neonate in an intensive care unit grew coagulase-negative staphylococci. This MOST likely indicates:
Laboratory error.
Contamination.
Colonization.
Infection.
The scenario involves a febrile neonate in an intensive care unit (ICU) with three out of four blood cultures growing coagulase-negative staphylococci (CoNS) over the past week. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes accurate interpretation of microbiological data in the "Identification of Infectious Disease Processes" domain, aligning with the Centers for Disease Control and Prevention (CDC) guidelines for healthcare-associated infections. Determining whether this represents a true infection, contamination, colonization, or laboratory error requires evaluating the clinical and microbiological context.
Option B, "Contamination," is the most likely indication. Coagulase-negative staphylococci, such as Staphylococcus epidermidis, are common skin flora and frequent contaminants in blood cultures, especially in neonates where skin preparation or sampling technique may be challenging. The CDC’s "Guidelines for the Prevention of Intravascular Catheter-Related Infections" (2017) and the Clinical and Laboratory Standards Institute (CLSI) note that multiple positive cultures (e.g., two or more) are typically required to confirm true bacteremia, particularly with CoNS, unless accompanied by clear clinical signs of infection (e.g., worsening fever, hemodynamic instability) and no other explanation. The inconsistency (three out of four cultures) and the neonate’s ICU setting—where contamination from skin or catheter hubs is common—suggest that the positive cultures likely result from contamination during blood draw rather than true infection. Studies, such as those in the Journal of Clinical Microbiology (e.g., Beekmann et al., 2005), indicate that CoNS in blood cultures is contaminated in 70-80% of cases when not supported by robust clinical correlation.
Option A, "Laboratory error," is possible but less likely as the primary explanation. Laboratory errors (e.g., mislabeling or processing mistakes) could occur, but the repeated growth in three of four cultures suggests a consistent finding rather than a random error, making contamination a more plausible cause. Option C, "Colonization," refers to the presence of microorganisms on or in the body without invasion or immune response. While CoNS can colonize the skin or catheter sites, colonization does not typically result in positive blood cultures unless there is an invasive process, which is not supported by the data here. Option D, "Infection," is the least likely without additional evidence. True CoNS bloodstream infections (e.g., catheter-related) in neonates are serious but require consistent positive cultures, clinical deterioration (e.g., persistent fever, leukocytosis), and often imaging or catheter removal confirmation. The febrile state alone, with inconsistent culture results, does not meet the CDC’s criteria for diagnosing infection (e.g., at least two positive cultures from separate draws).
The CBIC Practice Analysis (2022) and CDC guidelines stress differentiating contamination from infection to avoid unnecessary treatment, which can drive antibiotic resistance. Given the high likelihood of contamination with CoNS in this context, Option B is the most accurate answer.
Which of the following is the correct collection technique to obtain a laboratory specimen for suspected pertussis?
Cough plate
Nares culture
Sputum culture
Nasopharyngeal culture
The gold standard specimen for diagnosing pertussis (Bordetella pertussis infection) is a nasopharyngeal culture because:
B. pertussis colonizes the nasopharynx, making it the best site for detection.
A properly collected nasopharyngeal swab or aspirate increases diagnostic sensitivity.
This method is recommended for culture, PCR, or direct fluorescent antibody testing.
Why the Other Options Are Incorrect?
A. Cough plate – Not commonly used due to low sensitivity.
B. Nares culture – The nares are not a primary site for pertussis colonization.
C. Sputum culture – B. pertussis does not commonly infect the lower respiratory tract.
CBIC Infection Control Reference
APIC confirms that nasopharyngeal culture is the preferred method for diagnosing pertussis.
The infection preventionist observed a caregiver entering a room without performing hand hygiene. The BEST response would be to
post additional signage to remind caregivers to wash before entry.
provide immediate feedback and education to the caregiver.
install hand hygiene dispensers in more convenient areas.
design a unit-based education program.
Immediate feedback is a best practice in behavior correction and performance improvement. In hand hygiene non-compliance, real-time intervention allows for immediate correction, education, and reinforcement of infection prevention policies.
The APIC/JCR Workbook recommends:
“Provide simulation training… that provides immediate feedback—for example, how to properly insert a urinary catheter or perform hand hygiene.” This supports behavior change and staff learning.
The APIC Text emphasizes that real-time, direct feedback is more effective than passive measures like signage or delayed education campaigns.
The infection preventionist notes an increase in Clostridioides difficile infections (CDI) in the ICU. A Root Cause Analysis (RCA) is scheduled. What is the goal of a Root Cause Analysis?
Proactively identify potential failures.
Identify processes to prevent recurrence.
Determine strengths, weaknesses, opportunities, and threats.
Educate staff in order to avoid individual blame.
The CBIC Certified Infection Control Exam Study Guide (6th edition) defines a Root Cause Analysis (RCA) as a retrospective, systematic process used to understand why an adverse event or undesired outcome occurred and what system-level changes are needed to prevent it from happening again. In the context of an increase in Clostridioides difficile infections in an ICU, the primary goal of an RCA is to identify underlying process failures and implement corrective actions to prevent recurrence.
RCA focuses on systems and processes rather than individual performance. Through structured methods such as event mapping, cause-and-effect analysis, and contributing factor review, the team examines elements such as antimicrobial use, environmental cleaning practices, hand hygiene compliance, isolation implementation, diagnostic testing practices, and workflow design. The ultimate outcome of an RCA is a set of actionable, sustainable process improvements that reduce the likelihood of similar events in the future.
Option A describes Failure Mode and Effects Analysis (FMEA), which is a proactive risk assessment tool. Option C refers to a SWOT analysis, used for strategic planning rather than event investigation. Option D reflects an important principle of RCA culture (non-punitive), but it is not the primary goal.
For the CIC® exam, it is essential to recognize that the core purpose of RCA is preventing recurrence through system improvement, making option B the correct answer.
==========
Which of the following processes is essential for endoscope reprocessing?
Intermediate level disinfection and contact time
Pre-cleaning, leak testing, and manual cleaning
Inspection using a borescope and horizontal storage
Leak testing, manual cleaning, and low level disinfection
The correct answer is B, "Pre-cleaning, leak testing, and manual cleaning," as these processes are essential for endoscope reprocessing. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, proper reprocessing of endoscopes is critical to prevent healthcare-associated infections (HAIs), given their complex design and susceptibility to microbial contamination. The initial steps of pre-cleaning (removing gross debris at the point of use), leak testing (ensuring the endoscope’s integrity to prevent fluid ingress), and manual cleaning (using enzymatic detergents to remove organic material) are foundational to the reprocessing cycle. These steps prepare the endoscope for high-level disinfection or sterilization by reducing bioburden and preventing damage, as outlined in standards such as AAMI ST91 (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). Failure at this stage can compromise subsequent disinfection, making it a non-negotiable component of the process.
Option A (intermediate level disinfection and contact time) is an important step but insufficient alone, as intermediate-level disinfection does not achieve the high-level disinfection required for semi-critical devices like endoscopes, which must eliminate all microorganisms except high levels of bacterial spores. Option C (inspection using a borescope and horizontal storage) includes valuable quality control (inspection) and storage practices, but these occur later in the process and are not essential initial steps; vertical storage is often preferred to prevent damage. Option D (leak testing, manual cleaning, and low level disinfection) includes two essential steps (leak testing and manual cleaning) but is inadequate because low-level disinfection does not meet the standard for endoscopes, which require high-level disinfection or sterilization.
The emphasis on pre-cleaning, leak testing, and manual cleaning aligns with CBIC’s focus on adhering to evidence-based reprocessing protocols to ensure patient safety and prevent HAIs (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.4 - Implement environmental cleaning and disinfection protocols). These steps are mandated by guidelines to mitigate risks associated with endoscope use in healthcare settings.
Based on the scenarios, when should an infection preventionist suspect an outbreak?
Three positive routine environmental cultures of Staphylococcus aureus from the bone marrow transplant unit
Detection of three ventilator-associated pneumonia cases among patients in the intensive care unit (ICU) after updated case definition implementation
Increase in the number of Klebsiella pneumoniae carbapenemase–producing isolates in the ICU after implementation of new minimum inhibitory concentration breakpoints
Detection of three positive blood cultures with methicillin-resistant Staphylococcus aureus in the cardiac ICU for patients who underwent cardiac surgery in the same week
The Certification Study Guide (6th edition) emphasizes that an outbreak should be suspected when there is an unexpected clustering of infections by time, place, and person, particularly when cases share a common exposure or procedure. Option D meets all key criteria for outbreak suspicion: the same organism (methicillin-resistant Staphylococcus aureus), the same location (cardiac ICU), a common procedure (cardiac surgery), and a tight time frame (same week). This constellation strongly suggests possible transmission related to surgical practices, postoperative care, or shared equipment.
The other scenarios reflect situations that do not necessarily indicate an outbreak. Routine environmental cultures are not recommended for outbreak detection and often do not correlate with patient infection risk. An apparent increase in ventilator-associated pneumonia following implementation of a new case definition is likely due to surveillance artifact, not true transmission. Similarly, increases in carbapenemase-producing Klebsiella pneumoniae after adoption of new laboratory breakpoints reflect diagnostic changes, not an epidemiologic event.
The study guide stresses the importance of distinguishing true outbreaks from pseudo-outbreaks caused by changes in definitions, testing methods, or surveillance intensity. CIC exam questions frequently test this concept. Recognizing a true outbreak requires linking cases through epidemiologic characteristics—not simply increases in numbers.
Prompt recognition of true outbreaks enables timely investigation, implementation of control measures, and prevention of further transmission.
Given the formula for calculating incidence rates, the Y represents which of the following?

Population served
Number of infected patients
Population at risk
Number of events
Incidence rate is a fundamental epidemiological measure used to quantify the frequency of new cases of a disease within a specified population over a defined time period. The Certification Board of Infection Control and Epidemiology (CBIC) supports the use of such metrics in the "Surveillance and Epidemiologic Investigation" domain, aligning with the Centers for Disease Control and Prevention (CDC) "Principles of Epidemiology in Public Health Practice" (3rd Edition, 2012). The formula provided, XY×K=Rate\frac{X}{Y} \times K = RateYX×K=Rate, represents the standard incidence rate calculation, where KKK is a constant (e.g., 1,000 or 100,000) to express the rate per unit population, and the question asks what YYY represents among the given options.
In the incidence rate formula, XXX typically represents the number of new cases (or events) of the disease occurring during a specific period, and YYY represents the population at risk during that same period. The ratio XY\frac{X}{Y}YX yields the rate per unit of population, which is then multiplied by KKK to standardize the rate (e.g., cases per 1,000 persons). The CDC defines the denominator (YYY) as the population at risk, which includes individuals susceptible to the disease over the observation period. Option B ("Number of infected patients") might suggest XXX if it specified new cases, but as the denominator YYY, it is incorrect because incidence focuses on new cases relative to the at-risk population, not the total number of infected individuals (which could include prevalent cases). Option C ("Population at risk") correctly aligns with YYY, representing the base population over which the rate is calculated.
Option A, "Population served," is a broader term that might include the total population under care (e.g., in a healthcare facility), but it is not specific to those at risk for new infections, making it less precise. Option D, "Number of events," could align with XXX (new cases or events), but as the denominator YYY, it does not fit the formula’s structure. The CBIC Practice Analysis (2022) and CDC guidelines reinforce that the denominator in incidence rates is the population at risk, ensuring accurate measurement of new disease occurrence.
A construction project is completed at a healthcare facility and the construction barriers will be removed. Prior to using the space for patient care, an infection preventionist should recommend:
Sampling for airborne contaminants after construction.
Stocking supply rooms as soon as the rooms are available.
Testing the water for Legionella and other waterborne pathogens.
Inspecting and cleaning ducts if needed and balancing the ventilation system.
The Certification Study Guide (6th edition) emphasizes that restoration of a safe environment of care following construction or renovation is essential before patient occupancy. A primary concern after construction is the potential contamination and disruption of the heating, ventilation, and air conditioning (HVAC) system, which plays a critical role in infection prevention by controlling airflow, pressure relationships, and filtration.
Inspecting and cleaning air ducts as needed—and ensuring that the ventilation system is properly balanced—helps confirm that airflow is functioning as designed, including appropriate air exchanges, pressure differentials, and filtration efficiency. The study guide highlights that construction activities can introduce dust, debris, and microorganisms (including fungal spores) into ductwork, which may subsequently be disseminated into patient care areas if not addressed. Proper HVAC verification is a key component of post-construction clearance following an Infection Control Risk Assessment (ICRA).
The other options are not recommended as routine first steps. Air sampling is not advised because results are difficult to interpret and do not reliably predict infection risk. Stocking supplies before environmental clearance risks contamination of clean items. Routine water testing is not required unless water system disruption or stagnation occurred and is guided by a facility’s water management program rather than construction completion alone.
CIC exam questions frequently test post-construction readiness activities, reinforcing that HVAC inspection, cleaning, and balancing are critical prerequisites for safely reopening patient care spaces.
What method of evaluation will BEST identify a staff member’s competency with reprocessing medical devices?
Verbalize the importance of reprocessing.
Demonstrate the appropriate sterilization procedure.
Describe the facility’s sterilization policies and procedures.
Obtain a score of 100% on a post-test following a reprocessing course.
The correct answer is B, "Demonstrate the appropriate sterilization procedure," as this method of evaluation will best identify a staff member’s competency with reprocessing medical devices. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, competency in reprocessing medical devices—such as cleaning, disinfection, and sterilization—requires not only theoretical knowledge but also the practical ability to perform the tasks correctly and safely. Demonstration allows the infection preventionist (IP) to directly observe the staff member’s hands-on skills, adherence to protocols (e.g., AAMI ST79), and ability to handle equipment, ensuring that the reprocessing process effectively prevents healthcare-associated infections (HAIs) (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.3 - Assess competence of healthcare personnel). This method provides tangible evidence of proficiency, as it tests the application of knowledge in a real or simulated setting, which is critical for ensuring patient safety.
Option A (verbalize the importance of reprocessing) assesses understanding and awareness, but it is a theoretical exercise that does not confirm the ability to perform the task, making it insufficient for evaluating competency. Option C (describe the facility’s sterilization policies and procedures) tests knowledge of guidelines, which is a component of competence but lacks the practical demonstration needed to verify skill execution. Option D (obtain a score of 100% on a post-test following a reprocessing course) measures theoretical knowledge and retention, but a perfect score does not guarantee practical ability, as it does not assess hands-on performance or problem-solving under real conditions.
The focus on demonstration aligns with CBIC’s emphasis on assessing competence through observable performance, ensuring that staff can reliably reprocess devices to maintain a sterile environment (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This method supports a comprehensive evaluation, aligning with best practices for training and competency assessment in healthcare settings.
In a busy family practice clinic, a patient has been diagnosed with measles solely on the basis of their rash. Upon investigation, the infection preventionist (IP) learns the family waited for 20 minutes in the waiting room, unmasked. What is the IP’s NEXT step?
Contact Public Health
Start a contact tracing
Discuss necessary testing with provider
Confirm immunization status and presence of other symptoms
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that measles is a reportable, airborne disease, but actions such as public health notification and contact tracing should occur after appropriate clinical and laboratory confirmation is initiated, unless there is a clear epidemiologic link or high clinical suspicion.
In this scenario, the diagnosis was made solely on the basis of rash, which is insufficient to confirm measles. Many viral illnesses can present with rash, and misclassification can lead to unnecessary alarm, resource use, and disruption. Therefore, the next appropriate step for the infection preventionist is to discuss necessary diagnostic testing with the provider, such as measles-specific IgM serology and PCR testing, to confirm or rule out measles.
Options A and B are premature. Public health notification and contact tracing are essential after measles is suspected and testing is initiated or confirmed, but they should not precede diagnostic clarification when the diagnosis is uncertain. Option D may support clinical assessment but does not replace the need for laboratory confirmation.
The Study Guide highlights that infection preventionists must balance rapid response with diagnostic accuracy. Ensuring appropriate testing is initiated first allows subsequent infection control actions—such as airborne exposure assessment and public health reporting—to be targeted, evidence-based, and defensible.
For the CIC® exam, this question tests understanding of sequencing infection prevention actions, reinforcing that confirmation and testing discussion is the critical next step before escalation.
Though basic principles of emergency management remain the same for all types of disasters, which of the following interventions varies to address the specific needs of the situation?
Mitigation
Recovery
Response
Preparedness
The CBIC Certified Infection Control Exam Study Guide (6th edition) explains that emergency management is commonly described using four interrelated phases: mitigation, preparedness, response, and recovery. While all four phases are essential components of disaster management, the response phase is the intervention that varies the most depending on the specific type of disaster.
Response refers to the immediate actions taken during or directly after an event to protect life, contain hazards, and reduce further harm. These actions are highly situation-dependent. For example, the response to an infectious disease outbreak may involve isolation precautions, surge staffing, and antimicrobial management, whereas the response to a natural disaster may focus on evacuation, trauma care, and infrastructure stabilization. Because hazards differ widely in scope, transmission, severity, and resource needs, response activities must be tailored to the specific emergency.
Mitigation and preparedness are largely proactive and standardized, focusing on risk reduction and planning before an event occurs. Recovery also follows more predictable patterns, emphasizing restoration of services, evaluation, and long-term improvement. In contrast, response is dynamic and must be adapted in real time based on the nature, scale, and impact of the incident.
For the CIC® exam, this question tests understanding of emergency management frameworks. The key concept is that response activities are the most variable, making option C the correct answer.
An infection preventionist (IP) is reviewing blood cultures and notices several results with Arcanobacterium, coagulase-negative Staphylococcus, and Corynebacterium. What action is needed from the IP?
Disregard the results.
Call the Medical Staff Officer and declare there is an outbreak.
Work up each case as a healthcare-acquired bloodstream infection.
Collaborate with the lab manager to determine if there are trends or changes in practice.
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that certain organisms commonly recovered from blood cultures—such as Arcanobacterium, coagulase-negative Staphylococcus, and Corynebacterium—are frequently associated with skin contamination rather than true bloodstream infection. When multiple blood cultures yield these organisms, the infection preventionist must assess whether the findings represent contamination related to collection practices rather than immediately assuming infection or outbreak.
The most appropriate action is to collaborate with the laboratory manager and clinical teams to evaluate potential trends, specimen collection techniques, and changes in practice. This includes reviewing blood culture contamination rates, assessing skin antisepsis procedures, evaluating staff competency, and determining whether there has been an increase associated with a specific unit, shift, or collection method. Surveillance data and laboratory quality indicators are essential tools in this evaluation.
Option A is incorrect because results should never be disregarded without assessment. Option B is premature, as the organisms listed are not typical outbreak pathogens and require further analysis before escalation. Option C is inappropriate because these organisms do not automatically meet criteria for healthcare-associated bloodstream infection without supporting clinical evidence.
This scenario reflects a core CIC® exam concept: infection preventionists must apply epidemiologic principles, collaborate with laboratory services, and use data-driven analysis to differentiate contamination from infection and to guide quality improvement efforts.
=========
The BEST roommate selection for a patient with active shingles would be a patient who has had
varicella vaccine.
treatment with acyclovir
a history of herpes simplex.
varicclla zoster immunoglobulin
A patient with active shingles (herpes zoster) is contagious to individuals who have never had varicella (chickenpox) or the varicella vaccine. The best roommate selection is someone who has received the varicella vaccine, as they are considered immune and not at risk for contracting the virus.
Why the Other Options Are Incorrect?
B. Treatment with acyclovir – Acyclovir treats herpes zoster but does not prevent transmission to others.
C. A history of herpes simplex – Prior herpes simplex virus (HSV) infection does not confer immunity to varicella-zoster virus (VZV).
D. Varicella zoster immunoglobulin (VZIG) – VZIG provides temporary immunity but does not offer long-term protection like the vaccine.
CBIC Infection Control Reference
APIC guidelines recommend placing patients with active shingles in a room with individuals immune to varicella, such as those vaccinated.
Which of the following factors increases a patient’s risk of developing ventilator-associated pneumonia (VAP)?
Hypoxia
Nasogastric tube
Acute lung disease
In-line suction
Ventilator-associated pneumonia (VAP) is a type of healthcare-associated pneumonia that occurs in patients receiving mechanical ventilation for more than 48 hours. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes identifying risk factors for VAP in the "Prevention and Control of Infectious Diseases" domain, aligning with the Centers for Disease Control and Prevention (CDC) guidelines for preventing ventilator-associated events. The question requires identifying which factor among the options increases a patient’s risk of developing VAP, based on evidence from clinical and epidemiological data.
Option B, "Nasogastric tube," is the correct answer. The presence of a nasogastric tube is a well-documented risk factor for VAP. This tube can facilitate the aspiration of oropharyngeal secretions or gastric contents into the lower respiratory tract, bypassing natural defense mechanisms like the epiglottis. The CDC’s "Guidelines for Preventing Healthcare-Associated Pneumonia" (2004) and studies in the American Journal of Respiratory and Critical Care Medicine (e.g., Kollef et al., 2005) highlight that nasogastric tubes increase VAP risk by promoting microaspiration, especially if improperly managed or if the patient has impaired gag reflexes. This mechanical disruption of the airway’s protective barriers is a direct contributor to infection.
Option A, "Hypoxia," refers to low oxygen levels in the blood, which can be a consequence of lung conditions or VAP but is not a primary risk factor for developing it. Hypoxia may indicate underlying respiratory compromise, but it does not directly increase the likelihood of VAP unless associated with other factors (e.g., prolonged ventilation). Option C, "Acute lung disease," is a broad term that could include conditions like acute respiratory distress syndrome (ARDS), which may predispose patients to VAP due to prolonged ventilation needs. However, acute lung disease itself is not a specific risk factor; rather, it is the need for mechanical ventilation that elevates risk, making this less direct than the nasogastric tube effect. Option D, "In-line suction," involves a closed-system method for clearing respiratory secretions, which is designed to reduce VAP risk by minimizing contamination during suctioning. The CDC and evidence-based guidelines (e.g., American Thoracic Society, 2016) recommend in-line suction to prevent infection, suggesting it decreases rather than increases VAP risk.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize identifying modifiable risk factors like nasogastric tubes for targeted prevention strategies (e.g., elevating the head of the bed to reduce aspiration). Option B stands out as the factor most consistently linked to increased VAP risk based on clinical evidence.
Which of the following is the BEST strategy for reducing bloodstream infections associated with central venous catheters?
Routine replacement of central lines every 7 days.
Use of chlorhexidine-impregnated dressings.
Daily blood cultures for patients with central lines.
Use of povidone-iodine instead of chlorhexidine for skin antisepsis.
Chlorhexidine-impregnated dressings reduce central line-associated bloodstream infections (CLABSI) by preventing bacterial colonization.
Routine catheter replacement (A) increases insertion risks without reducing infections.
Daily blood cultures (C) are unnecessary and lead to false positives.
Povidone-iodine (D) is less effective than chlorhexidine for skin antisepsis.
CBIC Infection Control References:
APIC Text, "CLABSI Prevention Measures," Chapter 10.
A 22-year-old male has a splenectomy secondary to trauma. Which of the following vaccines is MOST important for this patient?
Haemophilus influenzae type B
Pneumococcal
Hepatitis B
Varicella
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that patients who have undergone splenectomy are at significantly increased risk for overwhelming postsplenectomy infection (OPSI), a rapidly progressive and potentially fatal condition. The spleen plays a critical role in clearing encapsulated organisms, and its absence markedly increases susceptibility to infections caused by these pathogens.
Among encapsulated bacteria, Streptococcus pneumoniae is the most common and most deadly cause of OPSI, making pneumococcal vaccination the single most important immunization for asplenic patients. Pneumococcal disease in individuals without a spleen can progress rapidly to sepsis, meningitis, or death, even in young and otherwise healthy adults. Therefore, ensuring pneumococcal vaccination—using the appropriate conjugate and polysaccharide vaccines according to age and immunization history—is a top priority.
While Haemophilus influenzae type B (Option A) and meningococcal vaccines are also recommended for asplenic patients, pneumococcal vaccination provides the greatest immediate protection against the most common cause of severe infection. Hepatitis B (Option C) and varicella (Option D) are important routine immunizations but are not specifically related to the increased infection risk associated with asplenia.
For the CIC® exam, it is critical to recognize that loss of splenic function necessitates prioritization of vaccines targeting encapsulated organisms, with pneumococcal vaccination being the most important.
A positive biological indicator is reported to the Infection Preventionist (IP) after a sterilizer was used. Which of the following should be done FIRST?
Check the Central Services employees' technique
Inform the risk manager of the positive indicator
Notify potentially affected patients of exposure to nonsterile equipment
Re-challenge the sterilizer with a second indicator
When a positive biological indicator (BI) is detected, the immediate response is to retest the sterilizer using another BI to confirm results. This helps distinguish between a true sterilization failure and a defective BI.
The CBIC Study Guide advises:
“If there is no indication of abnormalities, then the sterilizer should be tested again in three consecutive cycles using paired biological indicators from different manufacturers.”
Immediate recall is reserved for implant loads or confirmed sterilization failure.
Incorrect responses:
A. Check employee technique may be appropriate later but not as a first step.
B. Informing risk manager or C. Notifying patients occurs only after confirmation of failure.
An infection preventionist recommended incorporating the Mycobacterium tuberculosis (MTB) conversion rate as part of the facility’s annual risk assessment. Occupational Health provided the number of conversions among healthcare personnel (HCP) during the last year. Which additional information is needed to calculate the HCP conversion rate?
Number of HCP tested for MTB during the last year
Number of HCP that cared for patients with MTB in the last year
Number of HCP with unprotected exposure to patients with MTB in the last year
Number of HCP with positive tuberculin skin test or interferon gamma release assay in the last year
The Certification Study Guide (6th edition) defines the MTB conversion rate among healthcare personnel as a surveillance metric used in tuberculosis risk assessments to evaluate potential occupational exposure within a healthcare facility. A conversion represents a change from a previously negative TB screening test (such as a tuberculin skin test or interferon gamma release assay) to a positive result within a defined time period, typically one year.
To calculate a conversion rate, two elements are required: a numerator and a denominator. In this scenario, Occupational Health has already provided the numerator—the number of documented MTB conversions among HCP during the last year. The missing component is the denominator, which is the total number of HCP tested for MTB during that same time period. Without knowing how many personnel were screened, it is not possible to calculate a meaningful rate or trend.
The other options do not provide the appropriate denominator. Knowing how many HCP cared for TB patients or had unprotected exposures may inform risk evaluation but does not allow calculation of a rate. The number of HCP with positive tests reflects prevalence, not conversion, and does not account for baseline negative status.
The study guide emphasizes that accurate TB risk assessments rely on proper rate calculations, not raw counts. This concept is frequently tested on the CIC exam to ensure infection preventionists can correctly interpret occupational health surveillance data.
Which of the following control measures is MOST effective in preventing transmission of Legionella in healthcare water systems?
Flushing all faucets with hot water for 5 minutes daily.
Maintaining hot water storage temperatures above 140°F (60°C).
Installing carbon filters on all hospital water outlets.
Routine testing for Legionella in hospital water.
Maintaining hot water at 140°F (60°C) prevents Legionella growth and is the most effective control strategy.
Flushing water (A) alone is not sufficient.
Carbon filters (C) do not remove Legionella.
Routine testing (D) is not always necessary unless an outbreak occurs.
CBIC Infection Control References:
APIC Text, "Waterborne Pathogens and Infection Control," Chapter 9.
Endemic infection rate refers to
Disease that overwhelms the usual healthcare system.
The usual presence of a disease in a specific population or geographical area.
An outbreak of disease much greater than expected in a specific population or group.
The occurrence of more cases of a disease than expected in a given area or among a specific group.
The CBIC Certified Infection Control Exam Study Guide (6th edition) defines endemic infection rate as the constant or usual presence of a disease within a specific population, geographic area, or healthcare setting. An endemic level represents the baseline or expected frequency of disease occurrence over time, allowing infection preventionists to distinguish normal disease patterns from unusual increases that may signal outbreaks or epidemics.
Option B accurately reflects this definition by describing the expected and stable presence of a disease within a defined population or location. Endemic infections may persist at low or predictable levels and do not necessarily indicate a failure of infection prevention practices. Examples include seasonal influenza in the community or baseline rates of certain healthcare-associated infections within a facility.
Option A refers to a pandemic or healthcare system overload, not endemic disease. Options C and D describe outbreaks or epidemics, which involve a sudden increase in cases above the expected endemic level. These terms imply deviation from baseline and require investigation and intervention.
Understanding endemic rates is critical for infection prevention and surveillance because they provide the comparison point for identifying trends, clusters, and outbreaks. Surveillance data are interpreted against endemic baselines to determine whether changes reflect random variation or meaningful increases requiring action.
For the CIC® exam, recognizing epidemiologic terminology is essential. Endemic infection rate specifically refers to the usual or expected presence of disease, making option B the correct answer.
In the current year, cases of tuberculosis (TB) among foreign-born persons accounted for the majority of new TB cases in the United States. The number of states with greater than 50% of cases among foreign-born persons increased from four cases ten years ago to 22 cases in the current year. This information can BEST be used to
heighten awareness among Emergency Department staff.
inform staff who are foreign-born.
educate patients and visitors.
review the TB exposure control plan.
1 and 2 only.
1 and 4 only.
2 and 3 only.
3 and 4 only.
The correct answer is B, "1 and 4 only," indicating that the information can best be used to heighten awareness among Emergency Department (ED) staff and review the TB exposure control plan. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, tuberculosis (TB) remains a significant public health concern, particularly with the increasing proportion of cases among foreign-born persons in the United States. The data showing a rise from four to 22 states with over 50% of TB cases among foreign-born individuals highlights an evolving epidemiological trend that warrants targeted infection prevention strategies (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.1 - Conduct surveillance for healthcare-associated infections and epidemiologically significant organisms).
Heightening awareness among ED staff (option 1) is critical because the ED is often the first point of contact for patients with undiagnosed or active TB, especially those from high-prevalence regions. Increased awareness can improve early identification, isolation, and reporting of potential cases. Reviewing the TB exposure control plan (option 4) is equally important, as it allows the infection preventionist to assess and update protocols—such as ventilation, personal protective equipment (PPE) use, and screening processes—to address the heightened risk posed by the growing number of cases among foreign-born individuals (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.2 - Implement measures to prevent transmission of infectious agents).
Option 2 (inform staff who are foreign-born) is not the best use of this data, as the information pertains to patient demographics rather than staff risk, and targeting staff based on their origin could be inappropriate without specific exposure evidence. Option 3 (educate patients and visitors) is a general education strategy but less directly actionable with this specific epidemiological data, which is more relevant to healthcare worker preparedness and facility protocols. Combining options 1 and 4 aligns with CBIC’s emphasis on using surveillance data to guide prevention and control measures, ensuring a proactive response to the increased TB burden (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies).
An infection preventionist is developing training exercises for emergency preparedness and disaster response teams. The MOST effective instructional method for retaining information is:
Providing reading materials to the group.
Watching videos recorded by other hospitals.
Simulating an event to practice how to respond.
Administering a post-test after circulating the emergency response plan.
The Certification Study Guide (6th edition) emphasizes that active, experiential learning methods are the most effective for long-term retention of knowledge and skills, particularly in the context of emergency preparedness and disaster response. Simulation-based training allows participants to practice real-time decision-making, communication, and task execution in a controlled environment that closely mirrors actual emergency conditions.
Simulating an event—such as a mass casualty incident, infectious disease outbreak, or evacuation—engages learners cognitively, physically, and emotionally. The study guide notes that this type of hands-on training improves recall, reinforces correct behaviors, exposes system gaps, and builds team confidence. Simulation also supports interdisciplinary coordination and allows immediate feedback and debriefing, which further enhances learning retention.
The other instructional methods are less effective for retention. Reading materials and watching videos are passive learning approaches that may increase awareness but do not ensure competency during high-stress situations. Administering a post-test measures short-term knowledge acquisition but does not demonstrate the ability to apply that knowledge during an actual emergency.
CIC exam questions frequently highlight adult learning principles, stressing that people learn best by doing—especially when preparing for rare but high-risk events. Simulation-based exercises are therefore considered the gold standard for emergency preparedness training and are strongly recommended for disaster response teams.
Which of the following is included in an effective respiratory hygiene program in healthcare facilities?
Community educational brochures campaign
Mask availability at building entrance and reception
Separate entrance for symptomatic patients and visitors
Temperature monitoring devices at clinical unit entrance
An effective respiratory hygiene program in healthcare facilities aims to reduce the transmission of respiratory pathogens, such as influenza, COVID-19, and other droplet- or airborne infectious agents, by promoting practices that minimize the spread from infected individuals. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the importance of such programs within the "Prevention and Control of Infectious Diseases" domain, aligning with guidelines from the Centers for Disease Control and Prevention (CDC). The CDC’s "Guideline for Isolation Precautions" (2007) and its respiratory hygiene/cough etiquette recommendations outline key components, including source control, education, and environmental measures to protect patients, visitors, and healthcare workers.
Option B, "Mask availability at building entrance and reception," is a core element of an effective respiratory hygiene program. Providing masks at entry points ensures that symptomatic individuals can cover their mouth and nose, reducing the dispersal of respiratory droplets. This practice, often referred to as source control, is a primary strategy to interrupt transmission, especially in high-traffic areas like entrances and receptions. The CDC recommends that healthcare facilities offer masks or tissues and no-touch receptacles for disposal as part of respiratory hygiene, making this a practical and essential inclusion.
Option A, "Community educational brochures campaign," is a valuable adjunct to raise awareness among the public about respiratory hygiene (e.g., covering coughs, hand washing). However, it is an external strategy rather than a direct component of the facility’s internal program, which focuses on immediate action within the healthcare setting. Option C, "Separate entrance for symptomatic patients and visitors," can enhance infection control by segregating potentially infectious individuals, but it is not a universal requirement and depends on facility resources and design. The CDC suggests this as an optional measure during outbreaks, not a standard element of every respiratory hygiene program. Option D, "Temperature monitoring devices at clinical unit entrance," is a useful screening tool to identify febrile individuals, which may indicate infection. However, it is a surveillance measure rather than a core hygiene practice, and its effectiveness is limited without accompanying interventions like masking.
The CBIC Practice Analysis (2022) and CDC guidelines prioritize actionable, facility-based interventions like mask provision to mitigate transmission risks. The availability of masks at key entry points directly supports the goal of respiratory hygiene by enabling immediate source control, making Option B the most appropriate answer.
An infection preventionist is evaluating a new catheter that may decrease the rate of catheter-associated urinary tract infections. Which of the following provides the BEST information to support the selection of this catheter?
Staff member preference and product availability
Product materials and vendor information
Value analysis and information provided by the manufacturer
Cost benefit analysis and safety considerations
The correct answer is D, "Cost benefit analysis and safety considerations," as this provides the best information to support the selection of a new catheter aimed at decreasing the rate of catheter-associated urinary tract infections (CAUTIs). According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, selecting medical devices like catheters for infection prevention involves a comprehensive evaluation that balances efficacy, safety, and economic impact. A cost-benefit analysis assesses the financial implications (e.g., reduced infection rates leading to lower treatment costs) against the cost of the new catheter, while safety considerations ensure the device minimizes patient risk, such as reducing biofilm formation or irritation that contributes to CAUTIs (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.3 - Ensure safe reprocessing of medical equipment). This dual focus provides evidence-based data to justify the catheter’s adoption, aligning with the goal of improving patient outcomes and reducing healthcare-associated infections (HAIs).
Option A (staff member preference and product availability) is subjective and logistical rather than evidence-based, making it insufficient for a decision that impacts infection rates. Option B (product materials and vendor information) offers technical details but lacks the broader context of efficacy and cost-effectiveness needed for a comprehensive evaluation. Option C (value analysis and information provided by the manufacturer) includes a structured assessment of value, but it may be biased toward the manufacturer’s claims and lacks the independent safety and cost-benefit perspective critical for infection prevention decisions.
The emphasis on cost-benefit analysis and safety considerations reflects CBIC’s priority on using data-driven and patient-centered approaches to select interventions that enhance infection control (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.5 - Use data to guide infection prevention and control strategies). This approach ensures the catheter’s selection is supported by robust evidence, optimizing both clinical and economic outcomes in the prevention of CAUTIs.
A Quality Improvement Committee is trying to decrease catheter-associated urinary tract infections (CAUTIs) in the hospital. Which of the following would be an outcome measure that would help to show a reduction in CAUTIs?
Rate of patients receiving daily indwelling urinary catheter care
Percentage of patients with indwelling urinary catheters
Rate of CAUTI per 1000 indwelling urinary catheter days
Percentage of staff trained to insert indwelling urinary catheters
An outcome measure tracks the end result of healthcare processes. The CAUTI rate per 1,000 catheter days directly measures the frequency of infections, making it an ideal outcome metric.
From the APIC Text:
“An incidence rate (i.e., the number of new cases during a time period, such as the rate of patients with urinary catheters who get a CAUTI) is a frequently used outcome performance measure.”
Other choices like care compliance or training are process measures, not outcomes.
Which of the following factors influences the growth of microorganisms in a multi-dose medication vial?
Syringe size
Aseptic technique
Patient comorbidities
Administration techniques
The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies aseptic technique as the most critical factor influencing the growth of microorganisms in multi-dose medication vials. Multi-dose vials are designed for repeated entry and therefore carry an inherent risk of contamination if proper infection prevention practices are not strictly followed.
Microbial growth in a vial most often results from breaks in aseptic technique during medication preparation or access. This includes failure to disinfect the rubber septum with alcohol prior to vial entry, reuse of needles or syringes, use of contaminated hands or gloves, and improper storage after opening. Once microorganisms are introduced into a vial, preservatives may not fully inhibit growth, especially if contamination levels are high or storage conditions are suboptimal.
Syringe size (Option A) does not influence microbial growth. Patient comorbidities (Option C) affect infection risk in the patient but have no impact on contamination within the vial itself. Administration techniques (Option D) relate to how medication is delivered to the patient, not how organisms enter or proliferate within the medication container.
The Study Guide emphasizes that strict adherence to aseptic technique—including hand hygiene, use of sterile needles and syringes, septum disinfection, and proper storage—is essential to prevent contamination of multi-dose vials. Numerous healthcare-associated outbreaks have been traced to failures in these practices.
For the CIC® exam, this question reinforces that aseptic technique is the primary determinant of microbial contamination and growth in medication vials, making it the correct answer.
When conducting a literature search which of the following study designs may provide the best evidence of a direct causal relationship between the experimental factor and the outcome?
A case report
A descriptive study
A case control study
A randomized-controlled trial
To determine the best study design for providing evidence of a direct causal relationship between an experimental factor and an outcome, it is essential to understand the strengths and limitations of each study design listed. The goal is to identify a design that minimizes bias, controls for confounding variables, and establishes a clear cause-and-effect relationship.
A. A case report: A case report is a detailed description of a single patient or a small group of patients with a particular condition or outcome, often including the experimental factor of interest. While case reports can generate hypotheses and highlight rare occurrences, they lack a control group and are highly susceptible to bias. They do not provide evidence of causality because they are observational and anecdotal in nature. This makes them the weakest design for establishing a direct causal relationship.
B. A descriptive study: Descriptive studies, such as cross-sectional or cohort studies, describe the characteristics or outcomes of a population without manipulating variables. These studies can identify associations between an experimental factor and an outcome, but they do not establish causality due to the absence of randomization or control over confounding variables. For example, a descriptive study might show that a certain infection rate is higher in a group exposed to a specific factor, but it cannot prove the factor caused the infection without further evidence.
C. A case control study: A case control study compares individuals with a specific outcome (cases) to those without (controls) to identify factors that may contribute to the outcome. This retrospective design is useful for studying rare diseases or outcomes and can suggest associations. However, it is prone to recall bias and confounding, and it cannot definitively prove causation because the exposure is not controlled or randomized. It is stronger than case reports or descriptive studies but still falls short of establishing direct causality.
D. A randomized-controlled trial (RCT): An RCT is considered the gold standard for establishing causality in medical and scientific research. In an RCT, participants are randomly assigned to either an experimental group (exposed to the factor) or a control group (not exposed or given a placebo). Randomization minimizes selection bias and confounding variables, while the controlled environment allows researchers to isolate the effect of the experimental factor on the outcome. The ability to compare outcomes between groups under controlled conditions provides the strongest evidence of a direct causal relationship. This aligns with the principles of evidence-based practice, which the CBIC (Certification Board of Infection Control and Epidemiology) emphasizes for infection prevention and control strategies.
Based on this analysis, the randomized-controlled trial (D) is the study design that provides the best evidence of a direct causal relationship. This conclusion is consistent with the CBIC's focus on high-quality evidence to inform infection control practices, as RCTs are prioritized in the hierarchy of evidence for establishing cause-and-effect relationships.
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated guidelines, 2023), which emphasizes the use of high-quality evidence, including RCTs, for validating infection control interventions.
CBIC Examination Content Outline, Domain I: Identification of Infectious Disease Processes, which underscores the importance of evidence-based study designs in infection control research.
Based on the Spaulding classification, which of the following pairings is an example of a semi-critical item and its minimal level of disinfection?
Bedside table; high-level disinfection
Surgical instrument; sterilization
Endocavity probe; high-level disinfection
Bedpan; intermediate-level disinfection
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes the importance of applying Spaulding’s classification to determine the appropriate minimum level of processing for medical devices. Under this system, devices are categorized as critical, semi-critical, or noncritical based on the degree of infection risk associated with their use.
Semi-critical items are those that come into contact with mucous membranes or non-intact skin but do not ordinarily penetrate sterile tissue. Examples include endocavity probes, such as transvaginal or transrectal ultrasound probes. Because mucous membranes are more susceptible to infection than intact skin, semi-critical items require at least high-level disinfection after thorough cleaning to eliminate all microorganisms except large numbers of bacterial spores.
Option C correctly pairs an endocavity probe with high-level disinfection, which is the minimum acceptable level of processing for this classification. Option A is incorrect because a bedside table is a noncritical item and requires only low-level disinfection. Option B describes a critical item, which correctly requires sterilization but does not meet the question’s focus on semi-critical devices. Option D is incorrect because bedpans are noncritical items, and intermediate-level disinfection exceeds the minimum requirement.
Understanding Spaulding’s classification and matching devices to the correct level of disinfection is a high-yield topic on the CIC® exam and essential for safe infection prevention practice.
==========
Which of the following is an essential element of practice when sending biohazardous samples from one location to another?
Ship using triple-containment packaging
Electronically log and send via overnight delivery
Transport by an authorized biohazard transporter
Store in a cooler that is labeled as a health hazard
The safe transport of biohazardous samples, such as infectious agents, clinical specimens, or diagnostic materials, is a critical aspect of infection prevention and control to prevent exposure and environmental contamination. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes adherence to regulatory and safety standards in the "Prevention and Control of Infectious Diseases" domain, which includes proper handling and shipping of biohazardous materials. The primary guideline governing this practice is the U.S. Department of Transportation (DOT) Hazardous Materials Regulations (HMR) and the International Air Transport Association (IATA) Dangerous Goods Regulations, which align with global biosafety standards.
Option A, "Ship using triple-containment packaging," is the essential element of practice. Triple-containment packaging involves three layers: a primary watertight container holding the sample, a secondary leak-proof container with absorbent material, and an outer rigid packaging (e.g., a box) that meets shipping regulations. This system ensures that biohazardous materials remain secure during transport, preventing leaks or breaches that could expose handlers or the public. The CDC and WHO endorse this method as a fundamental requirement for shipping Category A (high-risk) and Category B (moderate-risk) infectious substances, making it the cornerstone of safe transport practice.
Option B, "Electronically log and send via overnight delivery," is a useful administrative and logistical step to track shipments and ensure timely delivery, but it is not the essential element. While documentation and rapid delivery are important for maintaining chain of custody and sample integrity, they are secondary to the physical containment provided by triple packaging. Option C, "Transport by an authorized biohazard transporter," is a necessary step to comply with regulations, as only trained and certified transporters can handle biohazardous materials. However, this is contingent on proper packaging; without triple containment, transport authorization alone is insufficient. Option D, "Store in a cooler that is labeled as a health hazard," may be part of preparation (e.g., maintaining sample temperature), but labeling alone does not address the containment or transport safety required during shipment. Coolers are often used, but the focus on labeling as a health hazard is incomplete without the triple-containment structure.
The CBIC Practice Analysis (2022) supports compliance with federal and international shipping regulations, which prioritize triple-containment packaging as the foundational practice to mitigate risks. The CDC’s Biosafety in Microbiological and Biomedical Laboratories (BMBL, 6th Edition, 2020) and IATA guidelines further specify that triple packaging is mandatory for all biohazardous shipments, reinforcing Option A as the correct answer.
Which of the following patients with human immunodeficiency virus infection requires Airborne precautions?
24-year-old male newly diagnosed with a CD4 count of 70
28-year-old female with Mycobacterium avium in sputum
36-year-old male with cryptococcal meningitis
46-year-old female with a cavitary lesion in upper lobe
HIV patients require Airborne Precautions if they have tuberculosis (TB). A cavitary lesion in the upper lobe is highly suggestive of active pulmonary TB, which requires Airborne Precautions due to aerosolized transmission.
Why the Other Options Are Incorrect?
A. 24-year-old male newly diagnosed with a CD4 count of 70 – Low CD4 count alone does not warrant Airborne Precautions unless there is active TB or another airborne pathogen.
B. 28-year-old female with Mycobacterium avium in sputum – Mycobacterium avium complex (MAC) is not airborne, and standard precautions are sufficient.
C. 36-year-old male with cryptococcal meningitis – Cryptococcus neoformans is not transmitted via the airborne route, so Airborne Precautions are unnecessary.
CBIC Infection Control Reference
Patients with HIV and suspected TB require Airborne Precautions until TB is ruled out.
Following an outbreak of Hepatitis A, the water supply is sampled. A high count of which of the following isolates would indicate that the water was a potential source?
Coliforms
Pseudomonads
Legionella
Acinetobacter
Coliform bacteria are indicators of fecal contamination in water, making them a critical measure of water safety. Hepatitis A is a virus primarily transmitted via the fecal-oral route, often through contaminated food or water.
Step-by-Step Justification:
Fecal Contamination and Hepatitis A:
Hepatitis A virus (HAV) spreads through ingestion of water contaminated with fecal matter. High coliform counts indicate fecal contamination and increase the risk of HAV outbreaks.
Use of Coliforms as Indicators:
Public health agencies use total coliforms and Escherichia coli (E. coli) as primary indicators of water safety because they signal fecal pollution.
Waterborne Transmission of Hepatitis A:
Hepatitis A outbreaks have been traced to contaminated drinking water, ice, and improperly treated wastewater. Coliform detection signals a need for immediate action.
Why Other Options Are Incorrect:
B. Pseudomonads:
Pseudomonads (e.g., Pseudomonas aeruginosa) are environmental bacteria but are not indicators of fecal contamination.
C. Legionella:
Legionella species cause Legionnaires' disease through inhalation of contaminated aerosols, not through fecal-oral transmission.
D. Acinetobacter:
Acinetobacter species are opportunistic pathogens in healthcare settings but are not indicators of waterborne fecal contamination.
CBIC Infection Control References:
APIC Text, "Water Systems and Infection Control Measures".
APIC Text, "Hepatitis A Transmission and Waterborne Outbreaks".
A laboratory has received specimens labeled eye drainage for four patients. In preparing an action plan, the infection preventionist should do which of the following FIRST?
Cohort the patients based on the presence of eye drainage.
Monitor hand-washing practices of staff and visitors.
Determine the location of the patients.
Conduct pulsed-field gel electrophoresis.
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that when a potential cluster of infections is identified, the first priority is situational awareness. Before implementing control measures or advanced laboratory analysis, the infection preventionist must determine whether the cases are epidemiologically linked. Identifying the location of the patients—such as whether they are on the same unit, service, or clinic—is the essential first step in assessing the likelihood of transmission or a common source.
Option C is correct because determining patient location allows the IP to evaluate spatial and temporal relationships, which form the foundation of outbreak investigation. If the patients are colocated, this may indicate shared staff, equipment, or environmental exposure, guiding immediate and targeted interventions.
Cohorting patients (Option A) is premature without confirming proximity or transmission risk. Monitoring hand hygiene (Option B) is an important control measure but should follow confirmation of potential spread or shared risk factors. Pulsed-field gel electrophoresis (Option D) is an advanced molecular typing method and is never an initial step; it is reserved for later stages when epidemiologic evidence suggests related cases.
For the CIC® exam, this question tests understanding of outbreak investigation sequencing. The Study Guide consistently reinforces that defining who, where, and when comes before interventions or laboratory typing, making determination of patient location the correct first action.
A patient has a draining sinus at the site of a left total hip arthroplasty. A culture from the sinus tract reveals four organisms. Which of the following specimens is optimal for identifying the eliologic agent?
Blood
Wound drainage
Joint aspirate
Sinus tract tissue
The optimal specimen for identifying the etiologic agent in a prosthetic joint infection (PJI) is a joint aspirate (synovial fluid). This is because:
It provides direct access to the infected site without contamination from external sources.
It allows for accurate microbiologic culture, Gram stain, and leukocyte count analysis.
Why the Other Options Are Incorrect?
A. Blood – Blood cultures may help detect hematogenous spread but are not the best sample for identifying localized prosthetic joint infections.
B. Wound drainage – Wound cultures often contain contaminants from surrounding skin flora and do not accurately reflect joint space infection.
D. Sinus tract tissue – Cultures from sinus tracts often represent colonization rather than the primary infecting organism.
CBIC Infection Control Reference
APIC guidelines confirm that joint aspirate is the most reliable specimen for diagnosing prosthetic joint infections.
In the Preparedness and Response Framework for Influenza Pandemics, intervals are used to describe an influenza pandemic progression. The interval “Deceleration of the Pandemic Wave” is characterized by:
Subject matter experts’ judgment of the potential implications for human health.
Identification of novel influenza A in humans or animals anywhere in the world.
Low pandemic influenza activity but continued possible outbreaks.
Consistently decreasing rate of pandemic influenza cases.
The CBIC Certified Infection Control Exam Study Guide (6th edition) describes the Preparedness and Response Framework for Influenza Pandemics as a structured model that divides a pandemic into distinct intervals to guide public health and healthcare response activities. These intervals include investigation, recognition, initiation, acceleration, deceleration, and preparation for future waves.
The Deceleration of the Pandemic Wave interval is defined by a consistent and sustained decrease in the number of new pandemic influenza cases, hospitalizations, and deaths. This decline reflects the impact of mitigation strategies such as vaccination campaigns, antiviral use, nonpharmaceutical interventions, and the development of population immunity. Although transmission is decreasing, healthcare systems are advised to remain vigilant, as localized transmission may still occur.
Option A describes activities associated with the Investigation Interval, when experts assess the potential public health implications of a novel virus. Option B corresponds to the Recognition Interval, marked by identification of a novel influenza A virus. Option C aligns more closely with the Preparation for Future Waves Interval, when overall activity is low but the risk of resurgence remains.
Understanding these distinctions is critical for infection preventionists, as response priorities shift during each interval. During deceleration, focus transitions from surge response to recovery planning, evaluation of response effectiveness, and preparation for potential subsequent waves—key concepts emphasized in the CIC® exam.
==========
`
To understand how their hospital-acquired infection rates compare to other health care settings, an infection preventionist (IP) plans to use benchmarking.
Which of the following criteria is important to ensure accurate benchmarking of surveillance data?
Data collectors are trained on how to collect data
Collecting data on a small population lo ensure accuracy of data collection
Denominator rates are selected based on an organizational risk assessment
Using case definitions that are adjusted for the patient population being studied
Benchmarking compares infection rates across healthcare facilities. For accurate benchmarking, case definitions must be standardized and adjusted for patient demographics, severity of illness, and other risk factors.
Why the Other Options Are Incorrect?
A. Data collectors are trained on how to collect data – Training is necessary, but it does not directly ensure comparability between facilities.
B. Collecting data on a small population – A larger sample size increases accuracy and reliability in benchmarking.
C. Denominator rates selected based on an organizational risk assessment – Risk assessment is important, but standardized case definitions are critical for comparison.
CBIC Infection Control Reference
According to APIC, accurate benchmarking relies on using standardized case definitions that account for differences in patient populations.
An infection preventionist (IP) receives a phone call from a local health department alerting the hospital of the occurrence of a sewer main break. Contamination of the city water supply is a possibility. Which of the following actions should the IP perform FIRST?
Notify the Emergency and Admissions departments to report diarrhea cases to infection control.
Review microbiology laboratory reports for enteric organisms in the past week.
Contact the Employee Health department and ask for collaboration in case-finding.
Review the emergency preparedness plan with engineering for sources of potable water.
The correct answer is B, "Review microbiology laboratory reports for enteric organisms in the past week," as this is the first action the infection preventionist (IP) should perform following the alert of a sewer main break and potential contamination of the city water supply. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, a rapid assessment of existing data is a critical initial step in investigating a potential waterborne outbreak. Reviewing microbiology laboratory reports for enteric organisms (e.g., Escherichia coli, Salmonella, or Shigella) helps the IP identify any recent spikes in infections that could indicate water supply contamination, providing an evidence-based starting point for the investigation (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.2 - Analyze surveillance data). This step leverages available hospital data to assess the scope and urgency of the situation before initiating broader actions.
Option A (notify the Emergency and Admissions departments to report diarrhea cases to infection control) is an important subsequent step to enhance surveillance, but it relies on proactive reporting and does not provide immediate evidence of an ongoing issue. Option C (contact the Employee Health department and ask for collaboration in case-finding) is valuable for involving additional resources, but it should follow the initial data review to prioritize case-finding efforts based on identified trends. Option D (review the emergency preparedness plan with engineering for sources of potable water) is a critical preparedness action, but it is more relevant once contamination is confirmed or as a preventive measure, not as the first step in assessing the current situation.
The focus on reviewing laboratory reports aligns with CBIC’s emphasis on using surveillance data to guide infection prevention responses, enabling the IP to quickly determine if the sewer main break has already impacted patient health and to escalate actions accordingly (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.1 - Conduct surveillance for healthcare-associated infections and epidemiologically significant organisms). This approach is consistent with CDC guidelines for responding to waterborne outbreak alerts (CDC Environmental Public Health Guidelines, 2020).
Which of the following blood-drawing methods is considered to be a needle-safe practice?
Use a syringe with a needle attached.
Shielded needles for vacuum-tube phlebotomy sets.
Remove contaminated needles from blood collection sets.
Inject blood into vacuum tubes using conventional syringes.
The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies engineered sharps injury prevention devices (ESIPDs) as the cornerstone of needle-safe practices during blood collection. Shielded needles used with vacuum-tube phlebotomy systems are specifically designed to reduce the risk of needlestick injuries by incorporating a built-in safety mechanism that covers or retracts the needle immediately after use.
Vacuum-tube systems with shielded needles allow blood to flow directly into collection tubes without the need for needle removal or blood transfer, thereby minimizing handling of sharps. Once blood collection is complete, the safety feature is activated—often automatically or with a single-handed technique—significantly reducing exposure risk to healthcare personnel. The Study Guide emphasizes that these devices meet regulatory expectations under the Needlestick Safety and Prevention Act and should be used whenever feasible.
The other options are unsafe practices. Using syringes with attached needles (Option A) increases risk during transfer and disposal. Removing contaminated needles from collection sets (Option C) is explicitly prohibited due to high injury risk. Injecting blood into vacuum tubes using conventional syringes (Option D) requires manipulating exposed needles and increases the likelihood of splashes and sharps injuries.
For CIC® exam preparation, it is essential to recognize that needle-safe blood collection relies on safety-engineered devices, with shielded vacuum-tube phlebotomy needles representing best practice for preventing occupational exposures.
During a COVID outbreak with hospital-associated transmission cases, the infection preventionist (IP) receives a news media call about what is being done to reduce the transmission. The IP's BEST response is to
answer the questions truthfully.
give vague answers to ensure patient privacy.
refer the reporters to the hospital's media spokesperson.
inform the reporter that the conversation must be recorded to ensure accuracy.
The best response for an infection preventionist (IP) when receiving a news media call during a COVID outbreak with hospital-associated transmission cases is to refer the reporters to the hospital's media spokesperson. This approach aligns with the principles outlined in the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, which emphasize the importance of maintaining professionalism, protecting patient privacy, and ensuring accurate communication. The IP's primary role is to focus on infection prevention and control activities rather than serving as a public relations representative. Engaging directly with the media can risk divulging sensitive patient information or operational details that may not be fully contextualized, potentially violating the Health Insurance Portability and Accountability Act (HIPAA) or other privacy regulations.
Option A (answer the questions truthfully) is not ideal because, while truthfulness is important, the IP may not have the authority or full context to provide a comprehensive and accurate public statement, and doing so could inadvertently compromise patient confidentiality or misrepresent hospital policies. Option B (give vague answers to ensure patient privacy) might protect privacy but could lead to miscommunication or lack of trust if the responses appear evasive without a clear referral process. Option D (inform the reporter that the conversation must be recorded to ensure accuracy) is a procedural step but does not address the core issue of who should handle media inquiries.
Referring to the hospital's media spokesperson (Option C) ensures that a trained individual handles the communication, adhering to CBIC's emphasis on collaboration with organizational leadership and adherence to institutional communication protocols (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.1 - Collaborate with organizational leaders). This also aligns with best practices for managing public health crises, where centralized and coordinated messaging is critical to avoid misinformation.
When assessing a patient’s infection prevention and control educational needs, it is necessary to FIRST determine the patient’s
severity of illness.
educational background.
duration of hospitalization.
baseline knowledge of the subject.
The correct answer is D, "baseline knowledge of the subject," as this is the necessary first step when assessing a patient’s infection prevention and control educational needs. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, effective patient education in infection prevention and control requires a tailored approach that begins with understanding the patient’s existing knowledge and comprehension of the topic. Determining baseline knowledge allows the infection preventionist (IP) to identify gaps, customize educational content to the patient’s level of understanding, and ensure the information is relevant and actionable (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.1 - Develop and implement educational programs). This step ensures that education is neither too basic nor overly complex, maximizing its effectiveness in promoting behaviors such as hand hygiene, wound care, or adherence to isolation protocols.
Option A (severity of illness) is an important clinical consideration that may influence the timing or method of education delivery, but it is not the first step in assessing educational needs. The severity might affect the patient’s ability to learn, but it does not directly inform the content or starting point of the education. Option B (educational background) provides context about the patient’s general learning capacity (e.g., literacy level or language preference), but it is secondary to assessing specific knowledge about infection prevention, as background alone does not reveal current understanding. Option C (duration of hospitalization) may impact the opportunity for education but is not a primary factor in determining what the patient needs to learn; it is more relevant to scheduling or prioritizing educational interventions.
The focus on baseline knowledge aligns with adult learning principles endorsed by CBIC, which emphasize assessing learners’ prior knowledge to build effective educational strategies (CBIC Practice Analysis, 2022, Domain IV: Education and Research, Competency 4.2 - Evaluate the effectiveness of educational programs). This approach ensures patient-centered care and supports infection control by empowering patients with the knowledge to participate in their own prevention efforts.
A healthy long-term employee with a history of Bacillus Calmette–Guérin (BCG) vaccination has a Tuberculin Skin Test (TST) result of 7 mm induration. The current Centers for Disease Control and Prevention (CDC) recommendations include which of the following?
Send the employee for a chest x-ray
No further action is required
Repeat the test in 1 to 3 weeks
Refer the employee to a physician for treatment
The CBIC Certified Infection Control Exam Study Guide (6th edition) aligns with CDC guidance regarding interpretation of the tuberculin skin test (TST) in healthcare personnel. For a healthy individual with no known risk factors for tuberculosis, a TST is considered positive only when induration is ≥10 mm. In this scenario, the employee’s TST result of 7 mm induration is negative and does not meet the threshold for latent TB infection.
A prior history of BCG vaccination does not change interpretation criteria in adults. The CDC explicitly recommends that TST results be interpreted regardless of BCG history, as vaccine-related reactivity typically wanes over time and induration should not be attributed to BCG alone. Therefore, a 7 mm reaction in a low-risk, asymptomatic healthcare worker does not require further diagnostic evaluation.
Option A (chest x-ray) is reserved for individuals with a positive TB test or symptoms suggestive of active TB. Option C (repeat testing) is not indicated unless this was part of a two-step baseline test and the first result was negative in a newly hired employee, which is not the case here. Option D is inappropriate because treatment is only considered after confirmed latent TB infection.
For the CIC® exam, it is essential to recognize that no further action is required when TST induration is below the positive threshold for the individual’s risk category, even in those with prior BCG vaccination.
An infection preventionist (IP) is asked to participate on a team to decrease ventilator-associated pneumonia (VAP) rates in a 20-bed ICU. The IP provides the following information. What is the first quarter ventilator utilization ratio?
Data Provided (First Quarter):
Ventilator days (Jan–Mar total): 800
Patient days (Jan–Mar total): 1200
0.13
0.67
1.50
1.67
The Certification Study Guide (6th edition) defines the ventilator utilization ratio (VUR) as a device utilization measure used in surveillance to describe the proportion of patient time during which a specific medical device—in this case, mechanical ventilation—is in use. It is calculated by dividing the total number of ventilator days by the total number of patient days for the same location and time period.
Using the first-quarter data provided, the calculation is as follows:
Ventilator Utilization Ratio = Ventilator Days ÷ Patient Days
Ventilator Utilization Ratio = 800 ÷ 1200 = 0.67
This means that ventilators were in use for 67% of all patient days in the ICU during the first quarter. The study guide emphasizes that device utilization ratios are essential for interpreting device-associated infection data, such as VAP rates, because they reflect the level of patient exposure to the device. Higher utilization increases the population at risk and can influence infection rates independently of prevention practices.
The other answer options are incorrect because they do not reflect the correct calculation. A ratio greater than 1.0 (options C and D) would imply more device days than patient days, which is not possible in this context. Option A underestimates utilization and does not match the provided data.
Understanding and correctly calculating utilization ratios is a core CIC exam competency, as these metrics support accurate surveillance, benchmarking, and performance improvement efforts.
A city has a population of 150.000. Thirty new cases of tuberculosis (TB) were diagnosed in the city last year. These now cases brought the total number of active TB cases in the city last year to 115. Which of the following equations represents the incidence rate tor TB per 100.000 in that year?
(30 ÷ 150.000) x 100.000 = X
(30÷ 150.000) x 100 = X
(115 ÷ 150.000) x 100.000 - X
(115 ÷ 100.000) x 100 = X
The incidence rate is calculated using the formula:
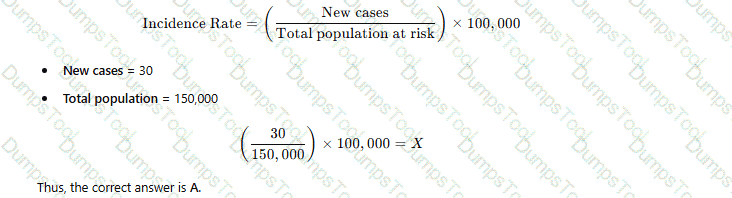
Why the Other Options Are Incorrect?
B. (30 ÷ 150,000) × 100 = X – Incorrect multiplier (should be 100,000 for standard incidence rate).
C. (115 ÷ 150,000) × 100,000 = X – 115 represents total cases (prevalence), not incidence.
D. (115 ÷ 100,000) × 100 = X – Uses the wrong denominator and multiplier.
CBIC Infection Control Reference
APIC defines the incidence rate as the number of new cases per population unit, typically per 100,000 people.
Which of the following community-acquired infections has the greatest potential public health impact?
Cryptosporidium enteritis
Fifth disease (parvovirus B-19)
Clostridial myositis (gas gangrene)
Cryptococcal meningitis
The correct answer is A, "Cryptosporidium enteritis," as it has the greatest potential public health impact among the listed community-acquired infections. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, the public health impact of an infection is determined by factors such as its transmissibility, severity, population at risk, and potential for outbreaks. Cryptosporidium enteritis, caused by the protozoan parasite Cryptosporidium, is a waterborne illness that spreads through contaminated water or food, leading to severe diarrhea, particularly in immunocompromised individuals. Its significant public health impact stems from its high transmissibility in community settings (e.g., via recreational water or daycare centers), the difficulty in eradicating the oocysts with standard chlorination, and the potential to cause large-scale outbreaks affecting vulnerable populations, such as children or the elderly (CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.3 - Apply principles of epidemiology). This is exemplified by notable outbreaks, such as the 1993 Milwaukee outbreak affecting over 400,000 people.
Option B (Fifth disease, caused by parvovirus B-19) is a viral infection primarily affecting children, causing a mild rash and flu-like symptoms. While it can pose risks to pregnant women (e.g., fetal anemia), it is generally self-limiting and has limited community-wide transmission potential, reducing its public health impact. Option C (clostridial myositis, or gas gangrene, caused by Clostridium perfringens) is a severe but rare infection typically associated with traumatic wounds or surgery, with limited person-to-person spread, making its public health impact low due to its sporadic nature. Option D (cryptococcal meningitis, caused by Cryptococcus neoformans) primarily affects immunocompromised individuals (e.g., those with HIV/AIDS) and is not highly transmissible in the general community, confining its impact to specific at-risk groups rather than the broader population.
The selection of Cryptosporidium enteritis aligns with CBIC’s focus on identifying infections with significant epidemiological implications, enabling infection preventionists to prioritize surveillance and control measures for diseases with high outbreak potential (CBIC Practice Analysis, 2022, Domain II: Surveillance and Epidemiologic Investigation, Competency 2.1 - Conduct surveillance for healthcare-associated infections and epidemiologically significant organisms). This is supported by CDC data highlighting waterborne pathogens as major public health concerns (CDC Parasites - Cryptosporidium, 2023).
There is an influenza epidemic in a community. To prevent transmission of influenza in a facility, the MOST rapidly effective measure an infection preventionist should recommend is to:
Use droplet precautions empirically for all residents suspected to have influenza.
Immediately immunize patient care staff.
Immunize patients and patient care staff.
Notify the local health department.
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that when influenza is circulating in the community, early recognition and immediate implementation of infection control measures are essential to prevent transmission within healthcare facilities. Among available interventions, the most rapidly effective measure is the empiric use of Droplet Precautions for patients suspected of having influenza.
Influenza is transmitted primarily through respiratory droplets generated by coughing, sneezing, or talking. Patients may be infectious before laboratory confirmation is available, making empiric precautions critical. Initiating Droplet Precautions—such as use of surgical masks, patient placement considerations, and limiting movement—immediately reduces the risk of person-to-person transmission and protects healthcare personnel and other patients.
While vaccination of staff and patients (Options B and C) is an essential long-term prevention strategy, it does not provide immediate protection because immunity develops over days to weeks. Therefore, vaccination alone is not the most rapidly effective intervention during an active outbreak. Option D, notifying the local health department, is important for surveillance and public health coordination but does not directly and immediately reduce transmission within the facility.
For CIC® exam preparation, it is crucial to distinguish between immediate containment measures and longer-term prevention strategies. Empiric Droplet Precautions for suspected influenza cases represent the fastest and most effective method to interrupt transmission during an influenza epidemic.
Which of the following is the BEST study design for assessing the benefit of a new treatment?
Interrupted time series
Correlational study
Parallel group study
Randomized controlled trial
The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies the randomized controlled trial (RCT) as the gold standard study design for assessing the benefit of a new treatment. RCTs are specifically designed to determine causality by minimizing bias and confounding variables through random assignment of participants to intervention and control groups. This ensures that differences in outcomes can be attributed with the highest level of confidence to the treatment being studied rather than to external factors.
In an RCT, participants are randomly allocated to receive either the new treatment or a comparison intervention (such as standard therapy or placebo). Randomization balances known and unknown risk factors between groups, while controlled conditions allow precise measurement of treatment effects. This design is particularly important when evaluating new therapies, medications, or interventions where efficacy and safety must be clearly demonstrated.
The other study designs listed are less rigorous for assessing treatment benefit. An interrupted time series is useful for evaluating system-level interventions over time but is more susceptible to confounding influences. A correlational study can identify associations but cannot establish cause and effect. A parallel group study without randomization lacks adequate control for bias and confounding.
For CIC® exam preparation, it is essential to recognize that when the objective is to assess the benefit or effectiveness of a new treatment, a randomized controlled trial provides the strongest and most reliable evidence, making it the best answer.
An adult with an incomplete vaccination history presents with an uncontrollable, rapid and violent cough, fever, and runny nose. Healthcare personnel should suspect
Pertussis.
Rhinovirus.
Bronchitis.
Adenovirus.
The correct answer is A, "Pertussis," as healthcare personnel should suspect this condition based on the presented symptoms and the patient’s incomplete vaccination history. According to the Certification Board of Infection Control and Epidemiology (CBIC) guidelines, pertussis, caused by the bacterium Bordetella pertussis, is characterized by an initial phase of mild respiratory symptoms (e.g., runny nose, low-grade fever) followed by a distinctive uncontrollable, rapid, and violent cough, often described as a "whooping" cough. This presentation is particularly concerning in adults with incomplete vaccination histories, as the pertussis vaccine’s immunity (e.g., DTaP or Tdap) wanes over time, increasing susceptibility (CBIC Practice Analysis, 2022, Domain I: Identification of Infectious Disease Processes, Competency 1.1 - Identify infectious disease processes). Pertussis is highly contagious and poses a significant risk in healthcare settings, necessitating prompt suspicion and isolation to prevent transmission.
Option B (rhinovirus) typically causes the common cold with symptoms like runny nose, sore throat, and mild cough, but it lacks the violent, paroxysmal cough characteristic of pertussis. Option C (bronchitis) may involve cough and fever, often due to viral or bacterial infection, but it is not typically associated with the rapid and violent cough pattern or linked to vaccination status in the same way as pertussis. Option D (adenovirus) can cause respiratory symptoms, including cough and fever, but it is more commonly associated with conjunctivitis or pharyngitis and does not feature the hallmark violent cough of pertussis.
The suspicion of pertussis aligns with CBIC’s emphasis on recognizing infectious disease patterns to initiate timely infection control measures, such as droplet precautions and prophylaxis for exposed individuals (CBIC Practice Analysis, 2022, Domain III: Infection Prevention and Control, Competency 3.2 - Implement measures to prevent transmission of infectious agents). Early identification is critical, especially in healthcare settings, to protect vulnerable patients and staff, and the incomplete vaccination history supports this differential diagnosis given pertussis’s vaccine-preventable nature (CDC Pink Book: Pertussis, 2021).
A family, including an infant of 8 months, is going on a vacation to Europe. An infection preventionist would recommend:
Exposure to rabies should be avoided.
Family members should be vaccinated for yellow fever.
The infant should not travel until at least 12 months of age.
Family immunization records should be reviewed by their provider.
When advising a family, including an 8-month-old infant, planning a vacation to Europe, an infection preventionist (IP) must consider travel-related health risks and vaccination recommendations tailored to the destination and age-specific guidelines. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the "Education and Training" domain, which includes providing evidence-based advice to prevent infections, aligning with the Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) travel health recommendations.
Option D, "Family immunization records should be reviewed by their provider," is the most appropriate recommendation. Europe, as a region, includes countries with varying health risks, but it is generally considered a low-risk area for many vaccine-preventable diseases compared to tropical regions. The CDC’s "Travelers’ Health" guidelines (2023) recommend that all travelers, including infants, have their immunization status reviewed by a healthcare provider prior to travel to ensure compliance with routine vaccinations (e.g., measles, mumps, rubella [MMR], diphtheria, tetanus, pertussis [DTaP], and polio) and to assess any destination-specific needs. For an 8-month-old, the review would confirm that the infant has received age-appropriate vaccines (e.g., the first doses of DTaP, Hib, PCV, and IPV, typically starting at 2 months) and is on schedule for the 6- and 12-month doses. This step ensures the family’s overall protection and identifies any gaps, making it a proactive and universally applicable recommendation.
Option A, "Exposure to rabies should be avoided," is a general travel safety tip applicable to any destination where rabies is endemic (e.g., parts of Eastern Europe or rural areas with wildlife). However, rabies risk in most European countries is low, and pre-exposure vaccination is not routinely recommended for travelers unless specific high-risk activities (e.g., handling bats) are planned. The CDC advises avoiding animal bites rather than vaccinating unless indicated, making this less specific and urgent than a records review. Option B, "Family members should be vaccinated for yellow fever," is incorrect. Yellow fever is not endemic in Europe, and vaccination is not required or recommended for travel to any European country. The WHO International Health Regulations (2005) and CDC list yellow fever vaccination as mandatory only for travelers from or to certain African and South American regions, rendering this irrelevant. Option C, "The infant should not travel until at least 12 months of age," lacks a clear evidence base. While some vaccines (e.g., MMR) are typically given at 12 months, the 8-month-old can travel safely if up-to-date on age-appropriate immunizations. The CDC allows travel for infants as young as 6 weeks with medical clearance, and delaying travel to 12 months is not a standard recommendation unless specific risks (e.g., disease outbreaks) are present, which are not indicated here.
The CBIC Practice Analysis (2022) and CDC Travelers’ Health resources prioritize pre-travel health assessments, including immunization reviews, as the foundation for safe travel. Option D ensures a comprehensive approach tailored to the family’s needs, making it the best recommendation for a trip to Europe.
A patient has an oral temperature of 101° F (38.33 C). Erythema and tenderness arc noted at the central line site. Blood samples are submitted for culture and intravenous vancomycin is ordered. This is an example of which of the following forms of antibiotic treatment?
Empiric
Prophylactic
Experimental
Broad spectrum
Empiric antibiotic therapy is the immediate initiation of antibiotics based on clinical judgment before laboratory confirmation of an infection. In this case, the presence of fever, erythema, and tenderness at the central line site suggests a possible bloodstream infection, prompting empiric treatment with vancomycin.
Step-by-Step Justification:
Initiation Before Lab Confirmation:
Empiric therapy starts treatment based on symptoms while awaiting culture results.
Prevents Complications:
Delayed treatment in central line-associated bloodstream infections (CLABSI) can lead to sepsis.
Common in High-Risk Situations:
Empiric treatment is used in cases where waiting for lab results could worsen the patient’s condition.
Why Other Options Are Incorrect:
B. Prophylactic:
Prophylactic antibiotics are given to prevent infection, not to treat an existing one.
C. Experimental:
Experimental treatment refers to clinical trials or unproven therapies, which does not apply here.
D. Broad spectrum:
Broad-spectrum antibiotics cover multiple bacteria, but empiric therapy may be narrow-spectrum based on suspected pathogens.
CBIC Infection Control References:
APIC Text, Chapter on Antimicrobial Stewardship and Empiric Therapy.
An infection preventionist is observing the cleaning and disinfection process of semi-critical devices. To ensure these items have been reprocessed meeting the minimum requirements, which of the following is required?
Use of detergents with pH lower than 7
Initial cleaning must begin 24 hours after use
Initial cleaning must begin as soon as possible after use
Soaking in a solution of liquid chemical sterilant between 3 and 12 hours
The Certification Study Guide (6th edition) emphasizes that thorough cleaning is the most critical step in the reprocessing of all reusable medical devices, including semi-critical devices (those that contact mucous membranes or nonintact skin). A foundational requirement is that initial cleaning begins as soon as possible after use. Prompt cleaning prevents organic material—such as blood, secretions, and tissue—from drying on device surfaces and within lumens, which can shield microorganisms and significantly reduce the effectiveness of subsequent disinfection.
The study guide explains that delayed cleaning increases the risk of biofilm formation and makes removal of soil more difficult, potentially compromising patient safety. For this reason, point-of-use pre-cleaning and rapid transport to reprocessing are considered minimum expectations. Cleaning must occur before any high-level disinfection or sterilization; without effective cleaning, even correctly selected disinfectants may fail.
The other options are incorrect or misleading. There is no universal requirement for detergents with pH lower than 7; detergent selection should follow manufacturer instructions. Waiting 24 hours before cleaning is contrary to best practice and increases risk. Soaking devices in liquid chemical sterilants for extended periods does not address the prerequisite of cleaning and may not be appropriate for semi-critical devices unless specified by the manufacturer.
This question reflects a key CIC exam principle: timely cleaning is non-negotiable and is the cornerstone of safe device reprocessing.
A patient presents with symptoms of meningitis. Two weeks ago, the patient ate from a cheese and meat tray and developed fever, muscle aches, and nausea the next day. Which of the following infectious agents should an infection preventionist consider as a cause of the patient’s symptoms?
Vibrio cholerae
Campylobacter jejuni
Clostridium perfringens
Listeria monocytogenes
The CBIC Certified Infection Control Exam Study Guide (6th edition) identifies Listeria monocytogenes as a significant foodborne pathogen capable of causing invasive disease, including meningitis. Listeria is uniquely associated with ready-to-eat foods, particularly soft cheeses, deli meats, pâtés, and meat trays—making the patient’s dietary history highly suggestive. Unlike many other foodborne organisms, Listeria can grow at refrigeration temperatures, increasing the risk of contamination in processed and stored foods.
The incubation period for invasive listeriosis can range from several days to weeks, which aligns with the timeline described. Early symptoms often include fever, myalgias, nausea, and gastrointestinal upset, followed by progression to meningitis or bloodstream infection, especially in high-risk populations such as older adults, pregnant individuals, neonates, and immunocompromised patients. The study guide emphasizes that Listeria is an important consideration when meningitis follows a compatible food exposure history.
The other organisms listed are primarily associated with self-limited gastrointestinal illness, not meningitis. Vibrio cholerae causes severe watery diarrhea; Campylobacter jejuni causes enteritis; and Clostridium perfringens causes toxin-mediated food poisoning with rapid onset diarrhea and abdominal cramping. None are typical causes of meningitis.
This question highlights a high-yield CIC exam concept: linking food exposure history to invasive pathogens, particularly Listeria monocytogenes, which requires prompt recognition and intervention.
==========
Which of the following findings indicates that a sputum sample has been properly collected from a patient with possible bacterial pneumonia?
Numerous neutrophils and few, if any, epithelial cells.
Presence of blood.
Many epithelial cells and few neutrophils.
Presence of both gram-positive and gram-negative bacteria.
The CBIC Certified Infection Control Exam Study Guide (6th edition) explains that the quality of a sputum specimen is critical for accurate diagnosis of bacterial pneumonia. A properly collected sputum sample should originate from the lower respiratory tract, not from saliva or the oropharynx. Microscopic examination of the specimen—typically using a Gram stain—is used to assess specimen adequacy before culture results are interpreted.
A high-quality sputum specimen is characterized by numerous neutrophils and few or no squamous epithelial cells. Neutrophils indicate an inflammatory response in the lower airways, consistent with bacterial infection. In contrast, epithelial cells originate from the mouth and upper respiratory tract; a large number of epithelial cells suggests contamination with saliva and an improperly collected specimen.
Option A correctly describes these criteria and therefore indicates proper specimen collection. Option C reflects poor-quality sputum contaminated with oral secretions and should be rejected or recollected. Option B (presence of blood) may occur in pneumonia but does not indicate specimen quality. Option D is nonspecific and may represent contamination or colonizing flora rather than true infection.
For the CIC® exam, it is important to recognize that specimen validity precedes interpretation of microbiologic results. The presence of abundant neutrophils with minimal epithelial cells confirms that the sputum sample is appropriate for diagnosing bacterial pneumonia and supports accurate clinical and epidemiologic decision-making.
The expectation to call out or speak up when an infection prevention lapse is observed is an example of
implementation of human factors.
honest disclosure of a safety event.
a blaming and shaming safety culture.
a safety culture with reciprocal accountability.
A safety culture with reciprocal accountability emphasizes mutual responsibility for maintaining safe practices, encouraging staff at all levels to "speak up" or "stop the line" when they observe risky practices. This concept reflects a learning organization and a just culture that supports open communication and proactive risk mitigation.
According to the APIC Text, a strong safety culture is described as one where:
“The leadership can expect staff members to call out or stop the line when they see risk, and staff can expect leadership to listen and act.”
This dynamic reflects reciprocal accountability.
Other options are less accurate:
A. Human factors refer to system design, not behavioral accountability.
B. Honest disclosure of a safety event is about post-event transparency, not real-time intervention.
C. A blaming and shaming culture is antithetical to safety culture principles.
Which of the following statements characterizes the proper use of chemical disinfectants?
All items to be processed must be cleaned prior to being submerged in solution.
The label on the solution being used must indicate that it kills all viable micro-organisms.
The solution should be adaptable for use as an antiseptic.
A chemical indicator must be used with items undergoing high-level disinfection.
The proper use of chemical disinfectants is a critical aspect of infection control, as outlined by the Certification Board of Infection Control and Epidemiology (CBIC). Chemical disinfectants are used to eliminate or reduce pathogenic microorganisms on inanimate objects, and their effective application requires adherence to specific protocols to ensure safety and efficacy. Let’s evaluate each option based on infection control standards:
A. All items to be processed must be cleaned prior to being submerged in solution.: This statement is a fundamental principle of disinfectant use. Cleaning (e.g., removing organic material such as blood, tissue, or dirt) is a prerequisite before disinfection because organic matter can inactivate or reduce the effectiveness of chemical disinfectants. The CBIC emphasizes that proper cleaning is the first step in the disinfection process to ensure that disinfectants can reach and kill microorganisms. This step is universally required for all levels of disinfection (low, intermediate, and high), making it a characterizing feature of proper use.
B. The label on the solution being used must indicate that it kills all viable micro-organisms.: This statement is misleading. No disinfectant can be guaranteed to kill 100% of all viable microorganisms under all conditions, as efficacy depends on factors like contact time, concentration, and the presence of organic material. Disinfectant labels typically indicate the types of microorganisms (e.g., bacteria, viruses, fungi) and the level of disinfection (e.g., high-level, intermediate-level) they are effective against, based on standardized tests (e.g., EPA or FDA guidelines). Claiming that a solution kills all viable microorganisms is unrealistic and not a requirement for proper use; instead, the label must specify the intended use and efficacy, which varies by product.
C. The solution should be adaptable for use as an antiseptic.: An antiseptic is a chemical agent used on living tissue (e.g., skin) to reduce microbial load, whereas a disinfectant is used on inanimate surfaces. While some chemicals (e.g., alcohol) can serve both purposes, this is not a requirement for proper disinfectant use. The adaptability of a solution for antiseptic use is irrelevant to its classification or application as a disinfectant, which focuses on environmental or equipment decontamination. This statement does not characterize proper disinfectant use.
D. A chemical indicator must be used with items undergoing high-level disinfection.: Chemical indicators (e.g., test strips or tapes) are used to verify that the disinfection process has met certain parameters (e.g., concentration or exposure time), particularly in sterilization or high-level disinfection (HLD). While this is a recommended practice for quality assurance in HLD (e.g., with glutaraldehyde or hydrogen peroxide), it is not a universal requirement for all chemical disinfectant use. HLD applies specifically to semi-critical items (e.g., endoscopes), and the need for indicators depends on the protocol and facility standards. This statement is too narrow and specific to characterize the proper use of chemical disinfectants broadly.
The correct answer is A, as cleaning prior to disinfection is a foundational and universally applicable step in the proper use of chemical disinfectants. This aligns with CBIC guidelines, which stress the importance of a clean surface to maximize disinfectant efficacy and prevent infection transmission in healthcare settings.
CBIC Infection Prevention and Control (IPC) Core Competency Model (updated 2023), Domain IV: Environment of Care, which mandates cleaning as a prerequisite for effective disinfection.
CBIC Examination Content Outline, Domain III: Prevention and Control of Infectious Diseases, which includes protocols for the proper use of disinfectants, emphasizing pre-cleaning.
CDC Guidelines for Disinfection and Sterilization in Healthcare Facilities (2021), which reinforce that cleaning must precede disinfection to ensure efficacy.
A nurse exposed to pertussis develops a mild cough 14 days later. What is the recommended action?
Continue working with a surgical mask.
Exclude from patient care until five days after starting antibiotics.
Initiate post-exposure prophylaxis only if symptoms worsen.
Conduct serologic testing before deciding on work restrictions.
The CDC recommends exclusion of healthcare workers with pertussis until completing at least five days of antibiotic therapy.
CBIC Infection Control References:
APIC-JCR Workbook, "Occupational Health Considerations," Chapter 10
The infection preventionist observes a nurse obtaining a wound culture and notes which of the following steps is correct?
The specimen is refrigerated to maintain integrity.
The nurse uses aseptic technique to collect the specimen.
The specimen container is labeled with the patient’s initials.
The specimen is obtained after the antibiotics have been started.
The CBIC Certified Infection Control Exam Study Guide (6th edition) emphasizes that aseptic technique is essential when obtaining clinical specimens, including wound cultures, to ensure accurate results and prevent contamination. Using aseptic technique minimizes the introduction of skin flora or environmental microorganisms that could lead to false-positive cultures and inappropriate clinical management.
Correct wound culture collection includes cleansing the wound as indicated, using sterile equipment, and avoiding contact with surrounding skin or nonsterile surfaces. This approach ensures that organisms identified in the culture are representative of true pathogens rather than contaminants. Proper specimen collection is a foundational infection prevention practice and directly affects diagnostic accuracy, antimicrobial stewardship, and patient outcomes.
Option A is incorrect because wound specimens are typically transported promptly at room temperature; refrigeration is not routinely recommended and may compromise certain organisms. Option C is incorrect because specimen containers must be labeled with at least two patient identifiers (such as full name and medical record number), not initials alone, to meet patient safety standards. Option D is incorrect because specimens should be obtained before initiation of antibiotic therapy whenever possible, as antibiotics can suppress bacterial growth and lead to false-negative results.
For CIC® exam preparation, it is critical to recognize that aseptic technique during specimen collection is the key correct practice, ensuring reliable laboratory results and supporting effective infection prevention and control efforts.
==========
A patient with suspected active tuberculosis is being transferred from a mental health facility to a medical center by emergency medical services. Which of the following should an infection preventionist recommend to the emergency medical technician (EMT)?
Place a surgical mask on both the patient and the EMT.
Place an N95 respirator on both the patient and the EMT.
Place an N95 respirator on the patient and a surgical mask on the EMT.
Place a surgical mask on the patient and an N95 respirator on the EMT.
Active tuberculosis (TB) is an airborne disease transmitted through the inhalation of droplet nuclei containing Mycobacterium tuberculosis. Effective infection control measures are critical during patient transport to protect healthcare workers, such as emergency medical technicians (EMTs), and to prevent community spread. The Certification Board of Infection Control and Epidemiology (CBIC) emphasizes the use of appropriate personal protective equipment (PPE) and source control as key strategies in the "Prevention and Control of Infectious Diseases" domain, aligning with guidelines from the Centers for Disease Control and Prevention (CDC).
For a patient with suspected active TB, the primary goal is to contain the infectious particles at the source (the patient) while ensuring the EMT is protected from inhalation exposure. Option C, placing an N95 respirator on the patient and a surgical mask on the EMT, is the most appropriate recommendation. The N95 respirator on the patient serves as source control by filtering the exhaled air, reducing the dispersion of infectious droplets. However, fitting an N95 respirator on the patient may be challenging, especially in an emergency setting or if the patient is uncooperative, so a surgical mask is often used as an alternative source control measure. For the EMT, a surgical mask provides a basic barrier but does not offer the same level of respiratory protection as an N95 respirator. The CDC recommends that healthcare workers, including EMTs, use an N95 respirator (or higher-level respiratory protection) when in close contact with a patient with suspected or confirmed active TB, unless an airborne infection isolation room is available, which is not feasible during transport.
Option A is incorrect because placing a surgical mask on both the patient and the EMT does not provide adequate respiratory protection for the EMT. Surgical masks are not designed to filter small airborne particles like those containing TB bacilli and do not meet the N95 standard required for airborne precautions. Option B is impractical and unnecessary, as placing an N95 respirator on both the patient and the EMT is overly restrictive and logistically challenging, especially for the patient during transport. Option D reverses the PPE roles, placing the surgical mask on the patient (insufficient for source control) and the N95 respirator on the EMT (appropriate for protection but misaligned with the need to control the patient’s exhalation). The CBIC and CDC guidelines prioritize source control on the patient and respiratory protection for the healthcare worker, making Option C the best fit.
This recommendation is consistent with the CBIC’s emphasis on implementing transmission-based precautions (CDC, 2005, Guideline for Preventing the Transmission of Mycobacterium tuberculosis in Healthcare Settings) and the use of PPE tailored to the mode of transmission, as outlined in the CBIC Practice Analysis (2022).
A surgical team is performing a liver transplant. Which of the following represents the HIGHEST risk for transmission of a healthcare-associated infection?
Failure to change surgical gloves after contamination.
Using alcohol-based hand rub instead of surgical scrub.
Delayed administration of preoperative antibiotics.
Airflow disruption due to personnel movement.
Glove Contamination and SSI Risk:
Failure to change contaminated gloves increases the risk of surgical site infections (SSIs).
Double-gloving with an outer glove change reduces contamination.
Why Other Options Are Incorrect:
B. Alcohol-based hand rubs: Are FDA-approved alternatives to traditional scrubs and effective.
C. Delayed antibiotics: Increases infection risk, but immediate correction reduces harm.
D. Airflow disruption: Can increase SSI risk, but glove contamination poses a more direct threat.
CBIC Infection Control References:
APIC-JCR Workbook, "Surgical Infection Prevention," Chapter 6.
Which of the following is the primary advantage of conducting prospective surveillance?
It is an efficient use of surveillance time.
It provides access to complete laboratory data.
It utilizes resources in a cost-effective manner.
It identifies clusters of infection in a timely manner.
The CBIC Certified Infection Control Exam Study Guide (6th edition) describes prospective surveillance as an active, real-time approach to infection surveillance in which patients are monitored as care is being delivered. The primary advantage of this method is its ability to identify infections, trends, and clusters promptly, allowing for early intervention and rapid implementation of control measures.
Because prospective surveillance occurs concurrently with patient care, infection preventionists can detect emerging patterns—such as an increase in device-associated infections or unusual organisms—before they become widespread outbreaks. This timely recognition supports immediate corrective actions, such as reinforcing isolation precautions, modifying clinical practices, or initiating focused investigations, thereby reducing transmission and patient harm.
The other options describe characteristics that are more consistent with retrospective surveillance. Option A and C are incorrect because prospective surveillance is typically more resource- and labor-intensive, not necessarily efficient or cost-effective. Option B is also incorrect because complete laboratory data may not yet be available in real time, whereas retrospective surveillance benefits from finalized records.
For the CIC® exam, it is important to understand that although prospective surveillance requires more resources, its key strength lies in early detection and timely response. The ability to quickly identify clusters of infection and intervene promptly is the defining advantage of prospective surveillance and the reason it is preferred for high-risk settings and priority infections.

TESTED 04 Mar 2026
Copyright © 2014-2026 DumpsTool. All Rights Reserved
